Targeting of IRAK4 and GSPT1 enhances therapeutic efficacy in AML via c-Myc destabilization
IF 13.4
1区 医学
Q1 HEMATOLOGY
引用次数: 0
Abstract
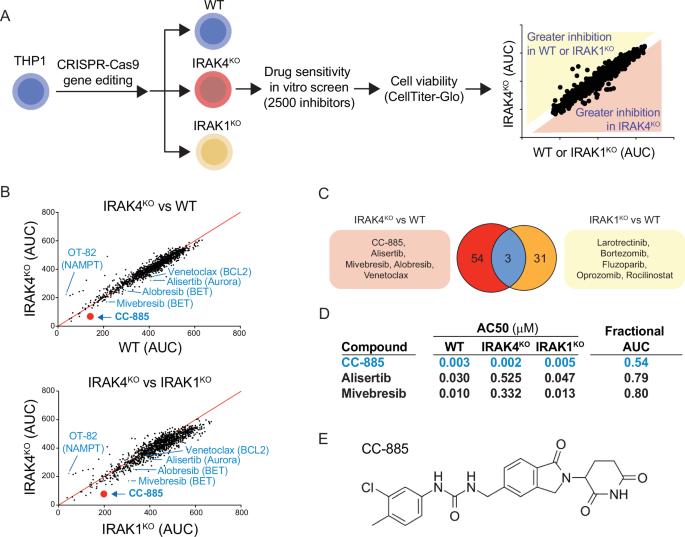
IRAK4 is a therapeutic target in myeloid malignancies, but current IRAK4 inhibitors show only modest clinical efficacy in acute myeloid leukemia, highlighting the need for combination strategies. To identify drugs with synergistic potential alongside IRAK4 inhibitors, we conducted a high-throughput screen of 2803 investigational and approved drugs in isogenic IRAK4-deficient and wild-type human AML cells. The top hit from this screen was the Cereblon E3 ligase modulator (CELMoD) CC-885. Validation in vitro and in vivo confirmed that CC-885 and related CELMoDs synergize with IRAK4 inhibitors to suppress leukemic cells. Among CC-885 substrates, GSPT1 loss showed the most pronounced effects in IRAK4-inhibited leukemic cells. Transcriptional and proteomic analyses revealed that CC-885 treatment led to c-Myc suppression in IRAK4-deficient leukemic cells. GSPT1 loss reduces translation efficiency, particularly for proteins with short half-lives, such as c-Myc. Accelerated c-Myc protein loss was confirmed following GSPT1 degradation in leukemic cells, with decreased protein stability observed following inhibition of IRAK4. These effects were validated in AML patient cells, supporting the potential of IRAK4 inhibitors to modulate c-Myc activity and enhance combinatorial therapies. This study demonstrates that IRAK4 is a therapeutic target in AML, and that combination therapies, such as with certain GSPT1-targeting CELMoDs, will be necessary to achieve maximal clinical responses.

靶向IRAK4和GSPT1通过破坏c-Myc的稳定性来增强AML的治疗效果
IRAK4是髓系恶性肿瘤的治疗靶点,但目前的IRAK4抑制剂在急性髓系白血病中仅显示出适度的临床疗效,这突出了联合策略的必要性。为了鉴定与IRAK4抑制剂具有协同潜力的药物,我们在等基因IRAK4缺陷和野生型人类AML细胞中对2803种正在研究和已批准的药物进行了高通量筛选。最受欢迎的是Cereblon E3连接酶调节剂(CELMoD) CC-885。体外和体内验证证实CC-885及相关CELMoDs与IRAK4抑制剂协同抑制白血病细胞。在CC-885底物中,GSPT1缺失对irak4抑制的白血病细胞的影响最为显著。转录和蛋白质组学分析显示,CC-885治疗导致irak4缺陷白血病细胞中c-Myc抑制。GSPT1的丢失降低了翻译效率,特别是对于半衰期较短的蛋白质,如c-Myc。在白血病细胞中,GSPT1降解后,c-Myc蛋白加速丢失,抑制IRAK4后,蛋白稳定性下降。这些作用在AML患者细胞中得到验证,支持IRAK4抑制剂调节c-Myc活性和增强联合治疗的潜力。这项研究表明,IRAK4是AML的治疗靶点,联合治疗,如与某些gspt1靶向的CELMoDs,将是实现最大临床反应的必要条件。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Leukemia
医学-血液学
CiteScore
18.10
自引率
3.50%
发文量
270
审稿时长
3-6 weeks
期刊介绍:
Title: Leukemia
Journal Overview:
Publishes high-quality, peer-reviewed research
Covers all aspects of research and treatment of leukemia and allied diseases
Includes studies of normal hemopoiesis due to comparative relevance
Topics of Interest:
Oncogenes
Growth factors
Stem cells
Leukemia genomics
Cell cycle
Signal transduction
Molecular targets for therapy
And more
Content Types:
Original research articles
Reviews
Letters
Correspondence
Comments elaborating on significant advances and covering topical issues
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


