Safety and tolerability of multiple sublingual microdoses of 5-MeO-DMT in adults with moderate symptoms of depression and/or anxiety: a randomized, double-blind, placebo-controlled study
IF 6.6
1区 医学
Q1 NEUROSCIENCES
引用次数: 0
Abstract
This Phase I clinical trial is the first to rigorously evaluate the safety, tolerability, and pharmacokinetics of a novel sublingual formulation of 5-MeO-DMT, administered at sub-psychedelic doses to adults with moderate to high levels of anxiety and/or depression, without formal psychiatric diagnosis or ongoing treatment. Using a double-blind, placebo-controlled design, participants received a single weekly sublingual dose of 5-MeO-DMT (6 mg, 9 mg, or 12 mg) or placebo over four weeks. The compound was well tolerated across all groups, with no significant adverse events or signs of organ toxicity; mild side effects such as nausea and headache were transient and self-resolving. Pharmacokinetic analyses showed rapid absorption, with peak plasma concentrations occurring within a median of 20 min and no evidence of drug accumulation. Neurophysiological assessments revealed dose-dependent modulation of brain activity without eliciting full psychedelic effects, supporting the feasibility of repeated sub-psychedelic dosing. Participants remained cognitively and behaviorally stable, maintaining their usual daily activities and social interactions. This study marks a pivotal advancement in the clinical exploration of psychedelic compounds, highlighting the potential of 5-MeO-DMT as a safe, fast-acting compound with favorable tolerability and emerging as a promising candidate for future therapeutic applications. These findings provide critical groundwork for future trials targeting psychiatric populations, positioning 5-MeO-DMT as a novel, fast-acting therapeutic strategy with broad clinical relevance. ClinicalTrials.gov: NCT06816667
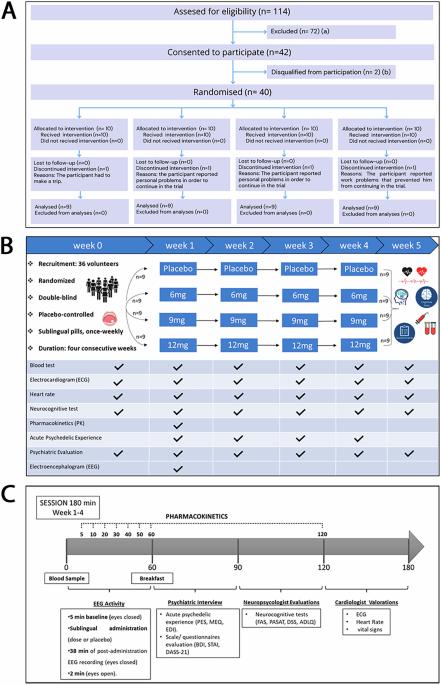
中度抑郁和/或焦虑症状成人多次舌下微剂量5-MeO-DMT的安全性和耐受性:一项随机、双盲、安慰剂对照研究
这项I期临床试验首次严格评估了5-MeO-DMT新型舌下配方的安全性、耐受性和药代动力学,该配方以亚迷幻剂量给药于患有中度至高度焦虑和/或抑郁的成年人,没有正式的精神诊断或正在接受治疗。采用双盲、安慰剂对照设计,参与者接受每周一次舌下剂量的5-MeO-DMT(6毫克、9毫克或12毫克)或安慰剂,为期四周。该化合物在所有组中耐受性良好,没有明显的不良事件或器官毒性迹象;轻微的副作用,如恶心和头痛是短暂的,并自行解决。药代动力学分析显示吸收迅速,血药浓度峰值在20分钟内出现,没有药物积累的证据。神经生理学评估显示,脑活动的剂量依赖性调节没有引起完全的迷幻效果,支持重复的亚迷幻剂量的可行性。参与者保持认知和行为稳定,维持日常活动和社会互动。这项研究标志着迷幻化合物临床探索的关键进展,突出了5-MeO-DMT作为一种安全、快速、耐受性良好的化合物的潜力,并有望成为未来治疗应用的候选者。这些发现为未来针对精神病人群的试验提供了重要的基础,将5-MeO-DMT定位为具有广泛临床相关性的新型快速治疗策略。试验注册:ClinicalTrials.gov: NCT06816667。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Neuropsychopharmacology
医学-精神病学
CiteScore
15.00
自引率
2.60%
发文量
240
审稿时长
2 months
期刊介绍:
Neuropsychopharmacology is a reputable international scientific journal that serves as the official publication of the American College of Neuropsychopharmacology (ACNP). The journal's primary focus is on research that enhances our knowledge of the brain and behavior, with a particular emphasis on the molecular, cellular, physiological, and psychological aspects of substances that affect the central nervous system (CNS). It also aims to identify new molecular targets for the development of future drugs.
The journal prioritizes original research reports, but it also welcomes mini-reviews and perspectives, which are often solicited by the editorial office. These types of articles provide valuable insights and syntheses of current research trends and future directions in the field of neuroscience and pharmacology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


