Functional evaluation of biotinylated Fab fragments after photochemical re-bridging with monobromomaleimide
IF 3
3区 医学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Re-bridging interchain disulfide bonds in antibody Fab fragments is essential for site-selective functionalization while preserving structural integrity. Here, we report a light-triggered [2 + 2] photocycloaddition strategy employing a biotinylated monobromomaleimide to covalently re-link reduced Fab fragments under mild conditions. While this photochemical reaction has been previously demonstrated in model systems, its impact on protein functionality remained untested. In this study, we applied the reaction to both polyclonal and monoclonal Fab fragments and verified successful re-bridging by non-reducing SDS-PAGE. Importantly, the conjugated Fabs retained their biotin-binding capability after UV irradiation, as confirmed by streptavidin-specific Western blotting. Antigen-binding activity was further validated by ELISA and SPR measurements. To our knowledge, this study is the first to demonstrate that photochemical disulfide re-bridging using monobromomaleimide derivatives can yield structurally and functionally competent Fab conjugates. This establishes a versatile platform for diagnostic and affinity-based applications.
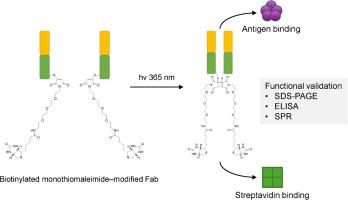
生物素化Fab片段与单溴代亚胺光化学桥接后的功能评价
重桥链间二硫键在抗体Fab片段是必要的位点选择性功能化,同时保持结构的完整性。在这里,我们报道了一种光触发的[2 + 2]光环加成策略,采用生物素化的单溴代亚胺在温和条件下共价重新连接还原的Fab片段。虽然这种光化学反应先前已在模型系统中得到证实,但其对蛋白质功能的影响尚未得到测试。在本研究中,我们将该反应应用于多克隆和单克隆Fab片段,并通过非还原SDS-PAGE验证了成功的桥接。重要的是,经链霉亲和素特异性Western blotting证实,偶联的fab在紫外线照射后仍保持其生物素结合能力。通过ELISA和SPR进一步验证抗原结合活性。据我们所知,这项研究首次证明了使用单溴代亚胺衍生物的光化学二硫重桥可以产生结构和功能上都有效的Fab偶联物。这为诊断和基于亲和力的应用程序建立了一个多功能平台。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Bioorganic & Medicinal Chemistry
医学-生化与分子生物学
CiteScore
6.80
自引率
2.90%
发文量
413
审稿时长
17 days
期刊介绍:
Bioorganic & Medicinal Chemistry provides an international forum for the publication of full original research papers and critical reviews on molecular interactions in key biological targets such as receptors, channels, enzymes, nucleotides, lipids and saccharides.
The aim of the journal is to promote a better understanding at the molecular level of life processes, and living organisms, as well as the interaction of these with chemical agents. A special feature will be that colour illustrations will be reproduced at no charge to the author, provided that the Editor agrees that colour is essential to the information content of the illustration in question.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


