Recapitulation of in vivo angiogenesis and osteogenesis within an ex vivo muscle pouch-based coral-derived macroporous construct organoid model
IF 5.9
1区 医学
Q1 ORTHOPEDICS
引用次数: 0
Abstract
Background
Segmental bone defect is a challenging clinical problem that often requires autologous bone grafting, which has limitations such as donor site morbidity and insufficient supply. Bone tissue engineering aims to create functional bone substitutes that can mimic the properties and processes of native bone. However, the discrepancy between in vitro and in vivo conditions hinders the successful translation of bone tissue engineering from animal models to human applications. Organoids, such as muscle pouch-based models, are emerging as promising tools that can closely resemble the osteogenic niche and overcome some of the limitations of conventional in vitro models.
Methods
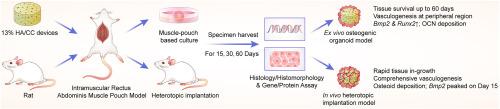
In this study, we explored two distinct muscle-biomaterial based bone induction models: an in vivo heterotopic implantation model and a novel ex vivo muscle pouch-based coral-derived macroporous construct organoid model. They both utilized the coral-derived constructs, specifically 13 % hydroxyapatite/calcium carbonate (13 % HA/CC) as the biomaterial. We implanted 72 coral-derived devices into rats' rectus abdominis muscle, divided equally between in vivo and ex vivo groups. Samples were harvested at 15, 30, and 60 days for molecular and histological analyses. We assessed the relative gene expression of angiogenesis markers (Vegfa and Col4a1) and osteogenesis signaling and structural markers (Runx2, Bmp2, Ocn and Alp) using qRT-PCR. We analyzed tissue morphogenesis, angiogenesis and induction of bone formation by H&E and modified Goldner's Trichrome staining. Immunostaining was further used to detect the expression and localization of OCN, VEGFA and CD31 in both in vivo and ex vivo models.
Results
We demonstrated that ex vivo muscle pouch-based coral-derived macroporous construct organoid model supported tissue survival up to 60 days with compromised tissue ingrowth compared to the in vivo model. Primary vascular structures formed at the tissue–scaffold interface in the organoid system with persistent up-regulation of Vegfa and Col4a1, while comprehensive angiogenesis took place with early up-regulation of Vegfa and Col4a1 in vivo. Proper bone formation was absent in both the ex vivo and in vivo models, but the in vivo models showed an up-regulation of Bmp2 and Alp in early phase and a delayed Ocn expression on day 30. The ex vivo model showed connective tissue formation, comprehensive OCN deposition, and gene expression patterns mimicking in vivo trends but with some distinctions.
Conclusions
The ex vivo muscle pouch-based coral-derived macroporous construct organoid model in this study can partially recapitulate angiogenesis and osteogenesis as compared to the in vivo model. However, key molecular signaling events that regulate these processes remained inactive. The study demonstrated that activating these events could enable the establishment of an ex vivo tissue-based vascularized model.
The translational potential of this article
This study partly elucidated the molecular signaling events involved in the development of an ex vivo tissue-based osteogenic organoid that closely resembled its in vivo counterpart. This would facilitate the development of well vascularized artificial bone grafts for treating segmental bone defects.
体外肌肉囊珊瑚衍生大孔构造类器官模型体内血管生成和骨生成的重现
背景节段性骨缺损是一个具有挑战性的临床问题,通常需要自体骨移植,但存在供体部位发病率和供体供应不足等局限性。骨组织工程旨在创造功能性骨替代品,可以模仿天然骨的特性和过程。然而,体外和体内条件之间的差异阻碍了骨组织工程从动物模型到人类应用的成功转化。类器官,如基于肌肉囊的模型,正在成为一种有前途的工具,它可以与成骨生态位非常相似,并克服了传统体外模型的一些局限性。方法在本研究中,我们探索了两种不同的基于肌肉生物材料的骨诱导模型:一种体内异位植入模型和一种新型的基于离体肌肉袋的珊瑚衍生大孔构建类器官模型。他们都使用了珊瑚衍生的结构,特别是13%羟基磷灰石/碳酸钙(13% HA/CC)作为生物材料。我们将72个珊瑚源性装置植入大鼠腹直肌,平均分为体内组和离体组。在15、30和60天采集样本进行分子和组织学分析。我们使用qRT-PCR评估血管生成标记(Vegfa和Col4a1)和成骨信号和结构标记(Runx2, Bmp2, Ocn和Alp)的相对基因表达。我们用H&;E和改良的三色染色分析组织形态发生、血管生成和骨形成诱导。采用免疫染色法检测OCN、VEGFA和CD31在体内和离体模型中的表达和定位。结果我们证明,与体内模型相比,基于离体肌肉袋的珊瑚衍生大孔构建类器官模型在组织生长受损的情况下支持组织存活长达60天。在类器官系统中,Vegfa和Col4a1持续上调可在组织-支架界面形成初级血管结构,而在体内,Vegfa和Col4a1早期上调可发生全面血管生成。离体和体内模型均未形成正常的骨,但体内模型在早期Bmp2和Alp表达上调,在第30天Ocn表达延迟。离体模型显示结缔组织形成、全面的OCN沉积和基因表达模式与体内趋势相似,但存在一些差异。结论与体内模型相比,本研究建立的离体肌袋珊瑚大孔结构类器官模型能部分再现血管生成和成骨过程。然而,调节这些过程的关键分子信号事件仍然不活跃。研究表明,激活这些事件可以建立基于离体组织的血管化模型。本研究部分阐明了体外组织类成骨器官发育过程中涉及的分子信号事件,该器官与体内类成骨器官非常相似。这将促进血管化良好的人工骨移植物的发展,用于治疗节段性骨缺损。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Orthopaedic Translation
Medicine-Orthopedics and Sports Medicine
CiteScore
11.80
自引率
13.60%
发文量
91
审稿时长
29 days
期刊介绍:
The Journal of Orthopaedic Translation (JOT) is the official peer-reviewed, open access journal of the Chinese Speaking Orthopaedic Society (CSOS) and the International Chinese Musculoskeletal Research Society (ICMRS). It is published quarterly, in January, April, July and October, by Elsevier.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


