Four-Component Selenoazaindolylation-Alkoxylation of Styrenes for the Synthesis of Azaindolyl β-Alkoxyalkyl Selenoethers
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
Due to the medicinal importance of selenoether and azaindole cores, the design and execution of efficient routes to new compounds bearing these groups placed in juxtaposition with each other are important. Again, multicomponent reactions provide fast and efficient access to the compounds of interest. Herein, we present a one-step route to azaindolyl β-alkoxyalkyl selenoethers or azaindolyl β-hydroxyalkyl selenoethers via the one-step reaction of azaindoles, styrenes, a selenium source, and an alcohol or water using a Ce(IV) oxidant.
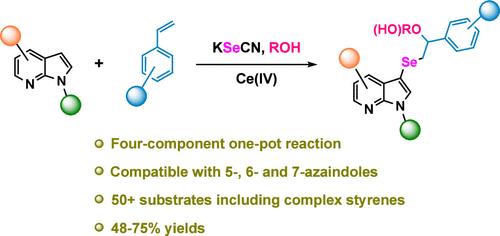
苯乙烯的四组分硒偶氮化-烷氧基化合成氮偶氮酰基β-烷氧烷基硒醚。
由于硒醚和再吲哚核心的药用重要性,设计和执行有效的路线,以新的化合物,这些基团放置在彼此并列是重要的。同样,多组分反应提供了快速有效地获得感兴趣的化合物的途径。在此,我们提出了一种一步制氮唑烯基β-烷氧烷基硒醚或氮唑烯基β-羟基烷基硒醚的方法,该方法通过氮唑烯、苯乙烯、硒源和乙醇或水在Ce(IV)氧化剂的作用下一步反应。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


