Metal- and base-free spirocyclization of alkylidene oxindoles via photo- and mechanochemically-generated nitrile ylides and nitrile imines as 1,3-dipoles†
Organic chemistry frontiers : an international journal of organic chemistry
Pub Date : 2025-04-28
DOI:10.1039/d5qo00851d
引用次数: 0
Abstract
Herein we have reported an expedient synthesis of spiro[pyrrolidine-3,3′-oxindole] and 2′-aryl-2′,4′-dihydrospiro[indoline-3,3′-pyrazol]-2-one under metal- and base-free conditions through the 3 + 2 cycloaddition reactions of in situ generated nitrile ylides and nitrile imines with alkylidene oxindoles in good to excellent yields. The nitrile ylides are generated through acetonitrile insertion onto carbenes generated from blue LED irradiation of aryl diazo esters. The nitrile imines were formed under mechanochemical conditions from diazo esters and aryl diazonium tetrafluoroborates.
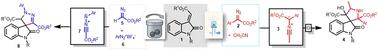
光化学和机械化学合成的腈酰亚胺和腈亚胺作为1,3偶极子的烷基二烯氧吲哚的金属和无碱螺旋环化反应
本文报道了在无金属和无碱条件下,通过原位生成的腈酰亚胺和腈酰亚胺与烷基酰氧吲哚进行3+2环加成反应,合成了螺[吡咯烷-3,3'-氧吲哚]和2'-芳基-2',4'-二氢螺[吲哚-3,3'-吡唑]-2- 1,收率较高。通过在芳基重氮酯的蓝光LED照射下生成的羰基上插入乙腈,生成了腈酰。以重氮酯和四氟硼酸重氮芳基为原料,在机械化学条件下合成了腈亚胺。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.80
自引率
0.00%
发文量
0

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


