Upadacitinib regulates pain-related pathways and BDNF expression in human monocyte-derived microglial-like cells
IF 7.6
2区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
Chronic pain is one of the most critical symptoms reported by patients with inflammatory arthritis, and even when joint inflammation improves, disabling residual pain may persist in a significant number of patients. Microglial cells, by producing different pro-inflammatory cytokines and pain-related molecules, including IL-1β and BDNF, are involved in neuroinflammation process. Treatment with Upadacitinib (upa), a JAK1 inhibitor, has been shown to be effective in improving disease activity and quickly relieving pain; however, the biological mechanisms underlying its efficacy against pain perception still require further investigation. This study aims to investigate whether and how upa may influence the production of pain and neuroinflammation-related molecules in pro-inflammatory human monocyte-derived microglia-like (M1-MDMi) model, specifically regarding BDNF. JAK1 inhibition by in vitro upa treatment downregulated BDNF expression and secretion by the modulation of P2X4 receptor, thus affecting a central mechanism involved in pain perception. Moreover, transcriptomic analysis showed that upa promoted an anti-nociceptive profile in the human glial model, by reducing the expression of neuroinflammatory, acute, and chronic pain-related pathways.
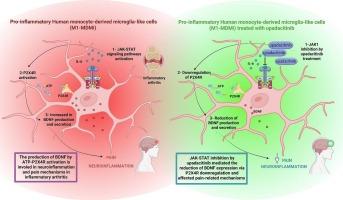
Upadacitinib调节人单核细胞源性小胶质样细胞的疼痛相关通路和BDNF表达。
慢性疼痛是炎症性关节炎患者报告的最关键的症状之一,即使关节炎症改善,残馀疼痛也可能持续存在于相当数量的患者中。小胶质细胞通过产生不同的促炎细胞因子和疼痛相关分子,包括IL-1β和BDNF,参与神经炎症过程。使用JAK1抑制剂Upadacitinib (upa)治疗已被证明在改善疾病活动性和快速缓解疼痛方面有效;然而,其对痛觉作用的生物学机制仍需进一步研究。本研究旨在探讨upa是否以及如何影响促炎人单核细胞源性小胶质细胞样(M1-MDMi)模型中疼痛相关和神经炎症相关分子的产生,特别是BDNF。体外upa处理抑制JAK1通过调节P2X4受体下调BDNF的表达和分泌,从而影响与疼痛感知有关的中枢机制。此外,转录组学分析表明,upa通过降低神经炎症、急性和慢性疼痛相关通路的表达,促进了人类神经胶质模型中的抗伤害性特征。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
29.60
自引率
2.00%
发文量
290
审稿时长
28 days
期刊介绍:
Established in 1987, Brain, Behavior, and Immunity proudly serves as the official journal of the Psychoneuroimmunology Research Society (PNIRS). This pioneering journal is dedicated to publishing peer-reviewed basic, experimental, and clinical studies that explore the intricate interactions among behavioral, neural, endocrine, and immune systems in both humans and animals.
As an international and interdisciplinary platform, Brain, Behavior, and Immunity focuses on original research spanning neuroscience, immunology, integrative physiology, behavioral biology, psychiatry, psychology, and clinical medicine. The journal is inclusive of research conducted at various levels, including molecular, cellular, social, and whole organism perspectives. With a commitment to efficiency, the journal facilitates online submission and review, ensuring timely publication of experimental results. Manuscripts typically undergo peer review and are returned to authors within 30 days of submission. It's worth noting that Brain, Behavior, and Immunity, published eight times a year, does not impose submission fees or page charges, fostering an open and accessible platform for scientific discourse.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


