Old mitochondria regulate niche renewal via α-ketoglutarate metabolism in stem cells
IF 20.8
1区 医学
Q1 ENDOCRINOLOGY & METABOLISM
引用次数: 0
Abstract
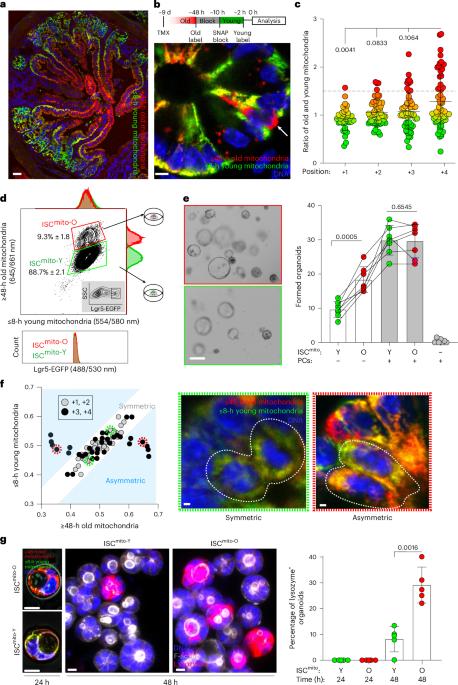
Cellular metabolism is a key regulator of cell fate1, raising the possibility that the recently discovered metabolic heterogeneity between newly synthesized and chronologically old organelles may affect stem cell fate in tissues2,3. In the small intestine, intestinal stem cells (ISCs)4 produce metabolically distinct progeny5, including their Paneth cell (PC) niche6. Here we show that asymmetric cell division of mouse ISCs generates a subset enriched for old mitochondria (ISCmito-O), which are metabolically distinct, and form organoids independently of niche because of their ability to recreate the PC niche. ISCmito-O mitochondria produce more α-ketoglutarate, driving ten-eleven translocation-mediated epigenetic changes that promote PC formation. In vivo α-ketoglutarate supplementation enhanced PC turnover and niche renewal, aiding recovery from chemotherapy-induced damage in aged mice. Our results reveal a subpopulation of ISCs whose old mitochondria metabolically regulate cell fate, and provide proof of principle for metabolically promoted replacement of specific aged cell types in vivo. Andersson et al. show that intestinal stem cells enriched for old mitochondria are metabolically distinct and have enhanced ability to regenerate the epithelial niche.

干细胞中衰老线粒体通过α-酮戊二酸代谢调节生态位更新
细胞代谢是细胞命运的关键调节因子1,这就提出了一种可能性,即最近发现的新合成细胞器和按时间顺序老化的细胞器之间的代谢异质性可能会影响组织中干细胞的命运2,3。在小肠中,肠干细胞(ISCs)产生代谢不同的后代5,包括它们的Paneth细胞(PC)壁龛6。在这里,我们发现小鼠ISCs的不对称细胞分裂产生了一个富集旧线粒体(iscmitto - o)的亚群,这是代谢独特的,并且由于它们能够重建PC生态位而独立于生态位形成类器官。ISCmito-O线粒体产生更多α-酮戊二酸,驱动10 - 11易位介导的表观遗传变化,促进PC的形成。体内补充α-酮戊二酸可促进老年小鼠PC细胞的更新和生态位更新,有助于化疗损伤后的恢复。我们的研究结果揭示了ISCs的一个亚群,其衰老的线粒体代谢调节细胞的命运,并为体内代谢促进特定衰老细胞类型的替换提供了原理证明。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature metabolism
ENDOCRINOLOGY & METABOLISM-
CiteScore
27.50
自引率
2.40%
发文量
170
期刊介绍:
Nature Metabolism is a peer-reviewed scientific journal that covers a broad range of topics in metabolism research. It aims to advance the understanding of metabolic and homeostatic processes at a cellular and physiological level. The journal publishes research from various fields, including fundamental cell biology, basic biomedical and translational research, and integrative physiology. It focuses on how cellular metabolism affects cellular function, the physiology and homeostasis of organs and tissues, and the regulation of organismal energy homeostasis. It also investigates the molecular pathophysiology of metabolic diseases such as diabetes and obesity, as well as their treatment. Nature Metabolism follows the standards of other Nature-branded journals, with a dedicated team of professional editors, rigorous peer-review process, high standards of copy-editing and production, swift publication, and editorial independence. The journal has a high impact factor, has a certain influence in the international area, and is deeply concerned and cited by the majority of scholars.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


