Investigating podophyllotoxin-induced cardiotoxicity in rats by Toxicological Evidence Chain (TEC): Focus on the Akt1/Srebp-1c/PUFAs axis
IF 5.4
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Podophyllotoxin, an efficacious constituent derived from the traditional Chinese medicine Dysosma versipellis, exhibits antitumor properties; however, its toxicity limits clinical application. This study, based on the Toxicological Evidence Chain (TEC), employs metabolomics and lipidomics approaches to investigate the mechanisms underlying its cardiotoxicity. Injury phenotype evidence (IPE) was obtained through observation of the rats’ external phenotypes, including coat condition, body weight, fecal characteristics, mental status, and presence of bleeding. Cardiac enzyme levels, blood lipid profiles, and histopathological examinations of heart tissues were assessed as evidence of adverse outcomes (AOE). To obtain evidence of toxic events, an integrated analysis of metabolomics, lipidomics, network toxicology was conducted on rat serum and heart tissues and molecular biology experiments in vitro, revealing the mechanism of PPT-induced cardiotoxicity. The results indicated that PPT induced pathological changes in rats, including weight loss, dull and brittle fur, and lethargy, along with nasal bleeding and diarrhea. The levels of AST, TC, and LDL-C were significantly elevated, while TG and HDL-C showed significant decreases. Integrated metabolomics analysis revealed 21 significantly altered differential metabolites demonstrating concordant directional changes across both serum and cardiac tissues. Complementary lipidomics profiling identified 31 dysregulated lipid species with coordinated variations in both biological matrices. These perturbed metabolites were principally associated with the core metabolic pathways: synthesis and metabolism of unsaturated fatty acid lipids. Network toxicology predictions identified Akt1, Egfr, Mtor, and Nos3 as potential critical molecular targets of PPT. In vitro experiments show that targeting the Akt1/Rebp-1c axis with PPT induces oxidative stress and cell death in cardiomyocytes, which can be mitigated by unsaturated fatty acids. This discovery offers a new approach for using PPT in clinical settings, potentially reducing its cardiotoxicity by administering unsaturated fatty acid simultaneously.
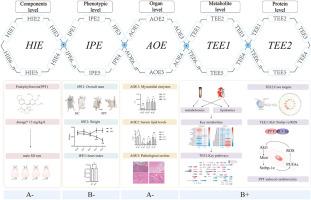
通过毒理学证据链(TEC)研究足叶毒素引起的大鼠心脏毒性:聚焦于Akt1/Srebp-1c/PUFAs轴
足臼毒素是一种有效成分,提取自传统中药水草,具有抗肿瘤作用;但其毒性限制了其临床应用。本研究基于毒理学证据链(TEC),采用代谢组学和脂质组学方法研究其心脏毒性的机制。通过观察大鼠的外部表型,包括皮毛状况、体重、粪便特征、精神状态和是否出血,获得损伤表型证据(IPE)。心脏酶水平、血脂谱和心脏组织的组织病理学检查被评估为不良结局(AOE)的证据。为了获得毒性事件的证据,我们对大鼠血清和心脏组织进行了代谢组学、脂质组学、网络毒理学的综合分析,并在体外进行了分子生物学实验,揭示了ppt诱导心脏毒性的机制。结果显示,PPT引起大鼠体重减轻、皮毛暗脆、嗜睡、鼻出血、腹泻等病理改变。AST、TC、LDL-C显著升高,TG、HDL-C显著降低。综合代谢组学分析显示21个显著改变的差异代谢物在血清和心脏组织中显示出一致的方向性变化。互补脂质组学分析鉴定了31种失调的脂质物种,它们在两种生物基质中具有协调的变异。这些紊乱的代谢物主要与核心代谢途径有关:不饱和脂肪酸脂质的合成和代谢。网络毒理学预测发现Akt1、Egfr、Mtor和Nos3是PPT潜在的关键分子靶点。体外实验表明,用PPT靶向Akt1/Rebp-1c轴可诱导心肌细胞氧化应激和细胞死亡,而不饱和脂肪酸可减轻这一过程。这一发现为临床应用PPT提供了一种新方法,通过同时施用不饱和脂肪酸,可能降低PPT的心脏毒性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.70
自引率
3.90%
发文量
410
审稿时长
36 days
期刊介绍:
Chemico-Biological Interactions publishes research reports and review articles that examine the molecular, cellular, and/or biochemical basis of toxicologically relevant outcomes. Special emphasis is placed on toxicological mechanisms associated with interactions between chemicals and biological systems. Outcomes may include all traditional endpoints caused by synthetic or naturally occurring chemicals, both in vivo and in vitro. Endpoints of interest include, but are not limited to carcinogenesis, mutagenesis, respiratory toxicology, neurotoxicology, reproductive and developmental toxicology, and immunotoxicology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


