Prostacyclin Assists in the Repair of Ruptured Amnions through the Proliferation and Migration of Amnion Mesenchymal Cells
IF 3.6
2区 医学
Q1 PATHOLOGY
引用次数: 0
Abstract
Preterm prelabor rupture of membrane (pPROM) is a risk factor for preterm birth. However, spontaneous healing of ruptured fetal membranes is occasionally clinically observed. Prostaglandins are involved in the wound-healing process in various tissues. Here, the role of prostacyclin (PGI2) in the repair of fetal membranes was investigated in a mouse model. Ptgs1, Ptgs2, Ptgis mRNA, and PGI2 were increased in the ruptured murine fetal membranes. Compared with the number of amnion mesenchymal cells at the intact site, the number of these cells at the rupture site was greater, and PGI2 synthase was increased in the mesenchymal cells of the amnion. Repair of the ruptured amnion was compromised by treatment with a PGI2 receptor (IP) antagonist, which decreased proliferation of the amnion mesenchymal cells. In contrast, an IP agonist partially restored repair of the amnion under the suppression of prostaglandin synthesis by a cyclooxygenase inhibitor. Compared with wild-type fetuses, IP-deficient fetuses exhibited impaired amnion repair, characterized by reduced proliferation of amnion mesenchymal cells observed at the rupture site. In vitro, the proliferation and migration of cultured human amnion mesenchymal cells were stimulated by an IP agonist and inhibited by an IP antagonist. These findings suggest that PGI2 facilitates amnion repair by promoting the proliferation and migration of amnion mesenchymal cells.
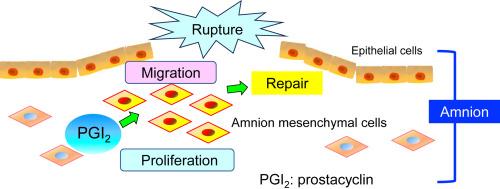
前列环素通过羊膜间充质细胞的增殖和迁移帮助修复破裂的羊膜。
早产胎膜破裂(pPROM)是早产的危险因素之一。然而,在临床上偶尔观察到胎膜破裂的自发愈合。前列腺素(pg)参与多种组织的伤口愈合过程。本研究在小鼠模型中研究了前列环素(PGI2)在胎膜修复中的作用。Ptgs1、Ptgs2、Ptgis mRNA和PGI2在破裂胎膜中表达升高。与完整部位相比,羊膜破裂部位羊膜间充质细胞数量较多,羊膜间充质细胞PGI2合成酶升高。PGI2受体(IP)拮抗剂会降低羊膜间充质细胞的增殖,从而破坏羊膜破裂的修复。相反,在COX抑制剂抑制PG合成的情况下,IP激动剂可以部分恢复羊膜的修复。与野生型胎儿相比,ip缺陷胎儿羊膜破裂的修复受到损害,破裂部位羊膜间充质细胞增殖较少。体外培养的人羊膜间充质细胞的增殖和迁移被一种IP激动剂刺激,而被一种IP拮抗剂抑制。这些发现表明PGI2通过促进羊膜间充质细胞的增殖和迁移来促进羊膜修复。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.40
自引率
0.00%
发文量
178
审稿时长
30 days
期刊介绍:
The American Journal of Pathology, official journal of the American Society for Investigative Pathology, published by Elsevier, Inc., seeks high-quality original research reports, reviews, and commentaries related to the molecular and cellular basis of disease. The editors will consider basic, translational, and clinical investigations that directly address mechanisms of pathogenesis or provide a foundation for future mechanistic inquiries. Examples of such foundational investigations include data mining, identification of biomarkers, molecular pathology, and discovery research. Foundational studies that incorporate deep learning and artificial intelligence are also welcome. High priority is given to studies of human disease and relevant experimental models using molecular, cellular, and organismal approaches.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


