Development of an explainable machine learning model for Alzheimer’s disease prediction using clinical and behavioural features
IF 1.9
Q2 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
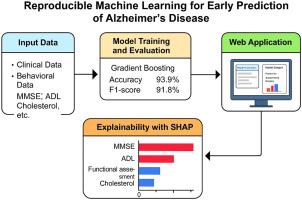
This article presents a reproducible machine learning methodology for the early prediction of Alzheimer’s disease (AD) using clinical and behavioural data. A comparative analysis of multiple classification algorithms was conducted, with the Gradient Boosting classifier yielding the best performance (accuracy: 93.9 %, F1-score: 91.8 %). To improve interpretability, SHapley Additive exPlanations (SHAP) were integrated into the workflow to quantify feature contributions at both global and individual levels. Key predictive variables such as Mini-Mental State Examination (MMSE), Activities of Daily Living (ADL), cholesterol levels, and functional assessment scores were identified and visualized using SHAP-based insights. A user-friendly, interactive web application was developed using Streamlit, allowing real-time patient data input and transparent model output visualization. This method offers a practical tool for clinicians and researchers to support early diagnosis and personalized risk assessment of AD, thus aiding in timely and informed clinical decision-making.
Accurate Prediction: Gradient Boosting model achieved 93.9 % accuracy for early Alzheimer’s detection.
Explainability: SHAP values provided interpretable insights into key clinical features.
Clinical Tool: A Streamlit-based web app enabled real-time, explainable predictions for users.
开发可解释的机器学习模型,用于使用临床和行为特征预测阿尔茨海默病
本文提出了一种可重复的机器学习方法,用于使用临床和行为数据进行阿尔茨海默病(AD)的早期预测。对多种分类算法进行了对比分析,其中Gradient Boosting分类器的准确率为93.9%,F1-score为91.8%,表现最佳。为了提高可解释性,SHapley加性解释(SHAP)被集成到工作流程中,以量化全局和个人层面的特征贡献。关键的预测变量,如迷你精神状态检查(MMSE)、日常生活活动(ADL)、胆固醇水平和功能评估分数,使用基于shap的见解进行识别和可视化。使用Streamlit开发了一个用户友好的交互式web应用程序,允许实时患者数据输入和透明的模型输出可视化。该方法为临床医生和研究人员提供了一种实用的工具,支持AD的早期诊断和个性化风险评估,从而有助于及时和知情的临床决策。准确预测:梯度增强模型对早期阿尔茨海默病的检测准确率达到93.9%。可解释性:SHAP值提供了对关键临床特征的可解释性见解。临床工具:一个基于流媒体的网络应用程序,为用户提供实时、可解释的预测。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

MethodsX
Health Professions-Medical Laboratory Technology
CiteScore
3.60
自引率
5.30%
发文量
314
审稿时长
7 weeks
期刊介绍:
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


