M6A RNA epitranscriptome dynamics linked to major depressive disorder and suicide risk
IF 6.6
1区 医学
Q1 NEUROSCIENCES
引用次数: 0
Abstract
Major depressive disorder (MDD) is the most prevalent psychiatric disorder. MDD patients are at substantially increased risk of dying by suicide. The molecular mechanisms associated with MDD and associated suicide are not clearly understood, which impedes the development of novel therapeutics. N6-methyladenosine (m6A) is the most prevalent epitranscriptomic mark on mRNA and plays significant roles in various physiological processes. This study investigated m6A RNA methylation and its potential contributions to MDD pathogenesis and associated suicide risk. High-throughput microarray analysis in the dorsolateral prefrontal cortex (dlPFC) of MDD subjects (n = 49) and non-psychiatric controls (n = 49) identified 1290 significantly hypermethylated and 6842 hypomethylated transcripts, with most m6A sites enriched in coding sequences. Chromosome-wide analysis showed hypermethylation hotspots on chromosomes 1 and 19. In-silico analysis identified enriched AAGA and ACCCA m6A motifs in the MDD group. Expression analysis revealed reduced FTO demethylase and increased METTL3 methyltransferase levels. A majority of M6A hypermethylated genes showed inverse correlation with their expression levels. Functional enrichment of hypermethylated genes highlighted disruptions in molecular pathways relevant to MDD. Comparison of MDD-non-suicide (n = 32) and MDD-suicide (n = 17) identified 6750 transcripts with significant hypermethylation, whereas 6159 transcripts had significant hypomethylation in the MDD-suicide group; of them, 196 hypermethylated genes were explicitly associated with suicide in the MDD group, whereas 38 hypermethylated genes appeared to elevate suicide risk in MDD patients. Also, the MDD-suicide group had distinct neuromolecular pathways associated with these risk genes. Collectively, the findings suggest a critical role for m6A methylation in modulating the molecular networks underlying MDD and suicide susceptibility.
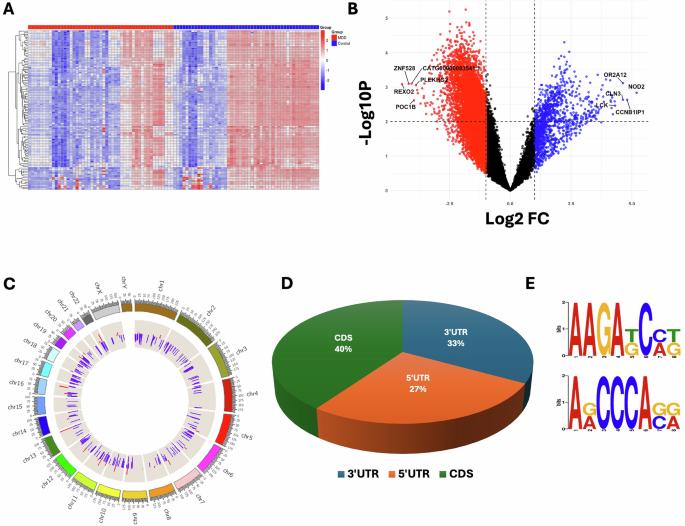
M6A RNA表转录组动力学与重度抑郁症和自杀风险相关。
重度抑郁症(MDD)是最常见的精神疾病。重度抑郁症患者死于自杀的风险大大增加。与重度抑郁症和相关自杀相关的分子机制尚不清楚,这阻碍了新疗法的发展。n6 -甲基腺苷(m6A)是mRNA上最普遍的表转录组标记,在各种生理过程中发挥重要作用。本研究探讨了m6A RNA甲基化及其在MDD发病机制和相关自杀风险中的潜在作用。在MDD受试者(n = 49)和非精神病学对照(n = 49)的背外侧前额叶皮质(dlPFC)中,高通量微阵列分析发现1290个显著高甲基化转录本和6842个显著低甲基化转录本,其中大多数m6A位点在编码序列中富集。全染色体分析显示1号和19号染色体上有高甲基化热点。芯片分析发现MDD组中富集了AAGA和acca m6A基序。表达分析显示FTO去甲基化酶降低,METTL3甲基转移酶水平升高。大多数M6A高甲基化基因与其表达水平呈负相关。高甲基化基因的功能富集突出了与MDD相关的分子通路的中断。比较mdd -非自杀组(n = 32)和mdd -自杀组(n = 17),发现6750个转录本显著高甲基化,而6159个转录本在mdd -自杀组中显著低甲基化;其中,196个高甲基化基因与重度抑郁症患者的自杀明显相关,而38个高甲基化基因似乎增加了重度抑郁症患者的自杀风险。此外,mdd自杀组具有与这些风险基因相关的独特神经分子通路。总的来说,这些发现表明m6A甲基化在调节MDD和自杀易感性的分子网络中起着关键作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Neuropsychopharmacology
医学-精神病学
CiteScore
15.00
自引率
2.60%
发文量
240
审稿时长
2 months
期刊介绍:
Neuropsychopharmacology is a reputable international scientific journal that serves as the official publication of the American College of Neuropsychopharmacology (ACNP). The journal's primary focus is on research that enhances our knowledge of the brain and behavior, with a particular emphasis on the molecular, cellular, physiological, and psychological aspects of substances that affect the central nervous system (CNS). It also aims to identify new molecular targets for the development of future drugs.
The journal prioritizes original research reports, but it also welcomes mini-reviews and perspectives, which are often solicited by the editorial office. These types of articles provide valuable insights and syntheses of current research trends and future directions in the field of neuroscience and pharmacology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


