DR1 activates histone gene expression to maintain pancreatic cancer cell survival through the ATAC complex
IF 5
3区 医学
Q1 BIOTECHNOLOGY & APPLIED MICROBIOLOGY
引用次数: 0
Abstract
Pancreatic ductal adenocarcinoma (PDAC) is the most malignant cancer and is characterized by short survival and limited treatment options. Epigenetic dysregulation is a defining feature of tumorigenesis but remains elusive in PDAC. Here, we identified DR1 as a vulnerability in PDAC. Loss of DR1 inhibited PDAC cell survival through repressing cell cycle. Mechanistically, DR1 recruited the ATAC complex to histone promoter regions to acetylate H3K9 and subsequently activate the expression of histone genes, ultimately promoting cell cycle and maintaining PDAC cell survival. Moreover, we uncovered a positive correlation between histone gene expression and the survival of patients with PDAC. In conclusion, our findings underscore the pivotal role of DR1 in the regulation of histone genes through the ATAC complex, providing a potential therapeutic target for PDAC.
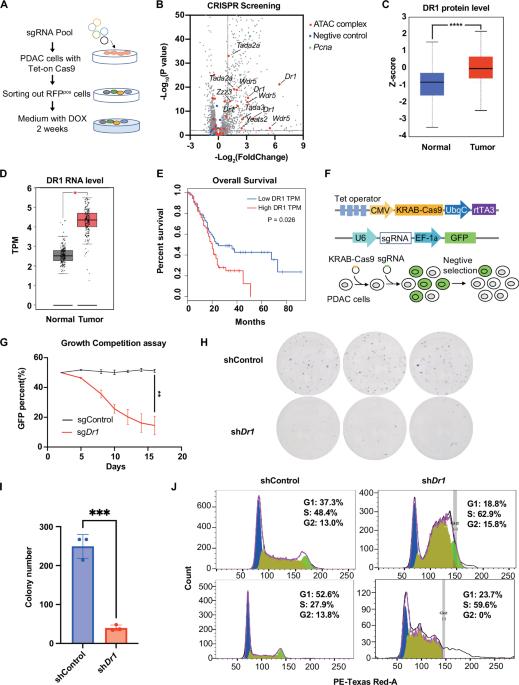
DR1通过ATAC复合物激活组蛋白基因表达,维持胰腺癌细胞存活。
胰腺导管腺癌(PDAC)是最恶性的癌症,其特点是生存期短,治疗选择有限。表观遗传失调是肿瘤发生的一个决定性特征,但在PDAC中仍然难以捉摸。在这里,我们将DR1确定为PDAC中的一个漏洞。DR1缺失通过抑制细胞周期抑制PDAC细胞存活。从机制上讲,DR1将ATAC复合物募集到组蛋白启动子区域,使H3K9乙酰化,随后激活组蛋白基因的表达,最终促进细胞周期,维持PDAC细胞存活。此外,我们发现组蛋白基因表达与PDAC患者的生存呈正相关。总之,我们的研究结果强调了DR1在通过ATAC复合物调节组蛋白基因中的关键作用,为PDAC提供了潜在的治疗靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cancer gene therapy
医学-生物工程与应用微生物
CiteScore
10.20
自引率
0.00%
发文量
150
审稿时长
4-8 weeks
期刊介绍:
Cancer Gene Therapy is the essential gene and cellular therapy resource for cancer researchers and clinicians, keeping readers up to date with the latest developments in gene and cellular therapies for cancer. The journal publishes original laboratory and clinical research papers, case reports and review articles. Publication topics include RNAi approaches, drug resistance, hematopoietic progenitor cell gene transfer, cancer stem cells, cellular therapies, homologous recombination, ribozyme technology, antisense technology, tumor immunotherapy and tumor suppressors, translational research, cancer therapy, gene delivery systems (viral and non-viral), anti-gene therapy (antisense, siRNA & ribozymes), apoptosis; mechanisms and therapies, vaccine development, immunology and immunotherapy, DNA synthesis and repair.
Cancer Gene Therapy publishes the results of laboratory investigations, preclinical studies, and clinical trials in the field of gene transfer/gene therapy and cellular therapies as applied to cancer research. Types of articles published include original research articles; case reports; brief communications; review articles in the main fields of drug resistance/sensitivity, gene therapy, cellular therapy, tumor suppressor and anti-oncogene therapy, cytokine/tumor immunotherapy, etc.; industry perspectives; and letters to the editor.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


