Novel synthesis of molybdenum phosphide employing a single source precursor and its use as a hydrogen evolution catalyst with broad pH activity
IF 3.3
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
Molybdenum phosphide is a promising noble-metal-free catalyst for the H2 evolution reaction. The synthesis of molybdenum phosphide was usually delicate, requiring a mixture of Mo and P precursors and hard reaction conditions. Herein, we report on a novel strategy for synthesizing molybdenum phosphide by employing a molybdenum complex, namely [MoIII(dppe)I3DMF] wherein dppe is 1,2-bis(diphenylphosphino) ethane, as the single precursor source. The synthesis, structural characterization and thermal behaviour of [MoIII(dppe)I3DMF], being a novel MoIII complex, was described. The thermal decomposition of [MoIII(dppe)I3DMF] generating hexagonal crystalline molybdenum phosphide was investigated in details showing the impacts of decomposition temperature to the product's morphology, chemical composition and crystallinity. The best molybdenum phosphide was obtained at 800 °C which showed attractive catalytic activities for the H2 evolution in acidic, neutral and alkaline electrolytes. It displayed an onset potential at −0.13 V vs. RHE and required −0.3 V vs. RHE to sustain the benchmarking catalytic current density of 10 mA/cm2. It was found to be stable during the H2 evolution catalysis.
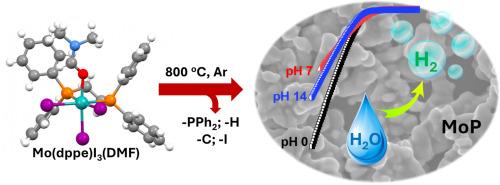
单源前驱体合成磷化钼的新方法及其作为宽pH活性析氢催化剂的应用
磷化钼是一种很有前途的无贵金属析氢催化剂。磷化钼的合成通常是精细的,需要Mo和P前驱体的混合物和苛刻的反应条件。本文报道了一种利用钼配合物[MoIII(dppe)I3DMF]合成磷化钼的新策略,其中dppe为1,2-二(二苯基膦)乙烷作为单一前驱体来源。报道了新型MoIII配合物[MoIII(dppe)I3DMF]的合成、结构表征和热行为。详细研究了[MoIII(dppe)I3DMF]热分解生成六方结晶磷化钼的过程,揭示了分解温度对产物形貌、化学成分和结晶度的影响。在800℃时得到了最佳的磷化钼,在酸性、中性和碱性电解质中表现出良好的析氢催化活性。与RHE相比,它的起始电位为- 0.13 V,需要- 0.3 V才能维持10 mA/cm2的基准催化电流密度。在析氢催化过程中,发现它是稳定的。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Solid State Sciences
化学-无机化学与核化学
CiteScore
6.60
自引率
2.90%
发文量
214
审稿时长
27 days
期刊介绍:
Solid State Sciences is the journal for researchers from the broad solid state chemistry and physics community. It publishes key articles on all aspects of solid state synthesis, structure-property relationships, theory and functionalities, in relation with experiments.
Key topics for stand-alone papers and special issues:
-Novel ways of synthesis, inorganic functional materials, including porous and glassy materials, hybrid organic-inorganic compounds and nanomaterials
-Physical properties, emphasizing but not limited to the electrical, magnetical and optical features
-Materials related to information technology and energy and environmental sciences.
The journal publishes feature articles from experts in the field upon invitation.
Solid State Sciences - your gateway to energy-related materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


