Combined downregulation of TGF-β1 and GRP78 is responsible for overcoming acquired sorafenib resistance, which is initiated by rewiring the cell surface CD44-GRP78-IGF-1R signaling circuit
IF 5
3区 医学
Q1 BIOTECHNOLOGY & APPLIED MICROBIOLOGY
引用次数: 0
Abstract
Previously, we showed that the downregulation of both HSP27 and TGF-β1 decreased the survival of various tumor types. However, we found that HSP27/TGF-β1 downregulation was less effective in acquired sorafenib-resistant HCC cell lines. As an alternative to HSP27/TGF-β1 downregulation to induce acute cell death in sorafenib-resistant cancer, we substituted shGRP78 for shHSP27 as a complement to shTGF-β1. The combination of shTGF-β1/shGRP78 was shown to overcome sorafenib resistance in HCC cell lines. Notably, both GRP78 and CD44 accumulate at the cell surface during sorafenib treatment and are accompanied by IRE1α activation; this effect is responsible for triggering and maintaining sorafenib resistance. These results revealed that sorafenib-induced acquired resistance in cancer cells is the result of receptor tyrosine kinase (RTK) feedback activation via the CD44-linked GRP78 signaling pathway with efficient rewiring of the GRP78-IGF1R-PI3K-Akt signaling cascade, which provides strong survival potential as well as a continuous positive feedback loop, resulting in sustained strong sorafenib resistance. In summary, CD44-GRP78 functions as both a sensor of sorafenib-induced ER stress and a mediator of sorafenib resistance.
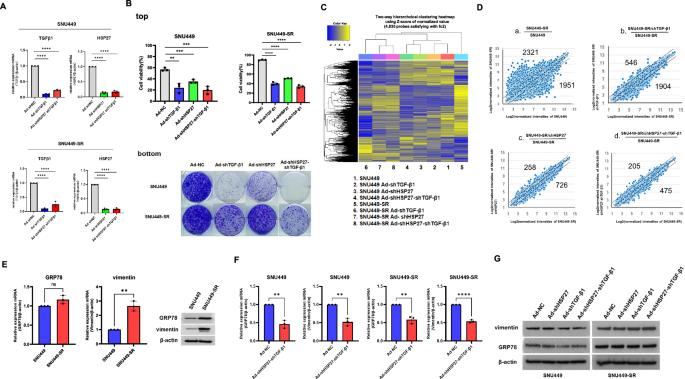
TGF-β1和GRP78的联合下调是克服获得性索拉非尼耐药的原因,这种耐药是通过重新连接细胞表面CD44-GRP78-IGF-1R信号通路而启动的。
之前我们发现HSP27和TGF-β1的下调降低了各种肿瘤类型的生存率。然而,我们发现HSP27/TGF-β1下调在获得性索拉非尼耐药HCC细胞系中效果较差。为了替代HSP27/TGF-β1下调诱导索拉非尼耐药癌症的急性细胞死亡,我们用shGRP78代替shHSP27作为shTGF-β1的补体。shTGF-β1/shGRP78联合使用可克服HCC细胞系索拉非尼耐药。值得注意的是,在索拉非尼治疗期间,GRP78和CD44都在细胞表面积累,并伴有IRE1α活化;这种作用是触发和维持索拉非尼耐药性的原因。这些结果表明,索拉非尼诱导的癌症细胞获得性耐药是受体酪氨酸激酶(RTK)通过cd44连接的GRP78信号通路反馈激活的结果,GRP78- igf1r - pi3k - akt信号级联的有效重新布线,提供了强大的生存潜力和持续的正反馈回路,导致持续的强索拉非尼耐药。综上所述,CD44-GRP78既是索拉非尼诱导内质网应激的传感器,也是索拉非尼耐药的介质。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cancer gene therapy
医学-生物工程与应用微生物
CiteScore
10.20
自引率
0.00%
发文量
150
审稿时长
4-8 weeks
期刊介绍:
Cancer Gene Therapy is the essential gene and cellular therapy resource for cancer researchers and clinicians, keeping readers up to date with the latest developments in gene and cellular therapies for cancer. The journal publishes original laboratory and clinical research papers, case reports and review articles. Publication topics include RNAi approaches, drug resistance, hematopoietic progenitor cell gene transfer, cancer stem cells, cellular therapies, homologous recombination, ribozyme technology, antisense technology, tumor immunotherapy and tumor suppressors, translational research, cancer therapy, gene delivery systems (viral and non-viral), anti-gene therapy (antisense, siRNA & ribozymes), apoptosis; mechanisms and therapies, vaccine development, immunology and immunotherapy, DNA synthesis and repair.
Cancer Gene Therapy publishes the results of laboratory investigations, preclinical studies, and clinical trials in the field of gene transfer/gene therapy and cellular therapies as applied to cancer research. Types of articles published include original research articles; case reports; brief communications; review articles in the main fields of drug resistance/sensitivity, gene therapy, cellular therapy, tumor suppressor and anti-oncogene therapy, cytokine/tumor immunotherapy, etc.; industry perspectives; and letters to the editor.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


