Mitochondrial apoptosis induced by MNAT1 in laryngeal squamous cell carcinoma cells reverses drug resistance
IF 5
2区 医学
Q2 Medicine
引用次数: 0
Abstract
Background
Laryngeal squamous cell carcinoma (LSCC), the predominant histological subtype of laryngeal cancer with a poor diagnosis, requires further exploration of its molecular mechanisms and potential therapeutic targets.
Methods
The expression of MNAT1 in LSCC was detected by western blotting and IHC. EDU analysis, colony formation assay, scratch assay, transwell assay and flow cytometry were used to detect cell proliferation, migration, invasion and apoptosis. The downstream genes of MNAT1 were predicted by RNA-seq. The interaction between MNAT1 and GDF15 was verified by Co-immunoprecipitation assay. The effect of MNAT1 on mitochondrial activity in LSCC cells was determined by ROS, JC-1, and lysosomal mitochondrial activity. The effect of MNAT1 and GDF15 on tumor growth of drug-resistant cells was evaluated in vivo.
Results
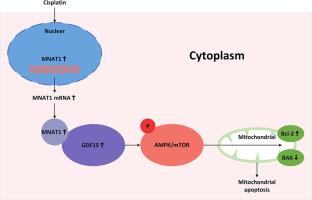
MNAT1 was highly expressed in LSCC tissues. After MNAT1-knockdown, the proliferation, migration and invasion of LSCC cells were inhibited, the level of apoptosis was significantly increased, and the resistance to cisplatin was decreased. MNAT1 interacts with GDF15. MNAT1 affects cell proliferation, migration and invasion through GDF15, and further affects mitochondrial apoptosis through AMPK pathway. In addition, MNAT1-knockdown and GDF15-knockdown reduced the tumor growth rate and enhanced the sensitivity of cisplatin in vivo.
Conclusions
MNAT1 promoted GDF15-mediated changes in AMPK pathway to affect mitochondrial apoptosis, which reveals the progression of LSCC and the mechanism of chemotherapy resistance, providing a new understanding of the mechanism of mitochondrial apoptosis and chemotherapy resistance in LSCC.
MNAT1诱导喉癌细胞线粒体凋亡逆转耐药
喉鳞状细胞癌(喉鳞状细胞癌)是喉癌的主要组织学亚型,诊断不佳,其分子机制和潜在的治疗靶点有待进一步探索。方法采用免疫印迹法和免疫组化法检测MNAT1在LSCC中的表达。采用EDU分析、菌落形成实验、划痕实验、transwell实验和流式细胞术检测细胞增殖、迁移、侵袭和凋亡。通过RNA-seq预测MNAT1的下游基因。MNAT1与GDF15的相互作用通过共免疫沉淀实验得到验证。MNAT1对LSCC细胞线粒体活性的影响通过ROS、JC-1和溶酶体线粒体活性测定。在体内评价MNAT1和GDF15对耐药细胞肿瘤生长的影响。结果smnat1在LSCC组织中高表达。mnat1敲低后,LSCC细胞的增殖、迁移和侵袭均受到抑制,凋亡水平显著升高,对顺铂的耐药性降低。MNAT1与GDF15相互作用。MNAT1通过GDF15影响细胞增殖、迁移和侵袭,并通过AMPK途径进一步影响线粒体凋亡。此外,mnat1敲低和gdf15敲低可降低肿瘤生长速度,提高体内顺铂的敏感性。结论smnat1促进gdf15介导的AMPK通路改变影响线粒体凋亡,揭示了LSCC的进展及化疗耐药机制,为LSCC线粒体凋亡及化疗耐药机制提供了新的认识。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Translational Oncology
ONCOLOGY-
CiteScore
8.40
自引率
2.00%
发文量
314
审稿时长
54 days
期刊介绍:
Translational Oncology publishes the results of novel research investigations which bridge the laboratory and clinical settings including risk assessment, cellular and molecular characterization, prevention, detection, diagnosis and treatment of human cancers with the overall goal of improving the clinical care of oncology patients. Translational Oncology will publish laboratory studies of novel therapeutic interventions as well as clinical trials which evaluate new treatment paradigms for cancer. Peer reviewed manuscript types include Original Reports, Reviews and Editorials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


