Synthesis and crystal structure analysis of bis(benzothiazole-2-thiolato-κS)(1,10-phenanthroline-κ2N,N′)zinc(II)
IF 0.6
Q4 CRYSTALLOGRAPHY
Acta Crystallographica Section E: Crystallographic Communications
Pub Date : 2025-07-01
DOI:10.1107/S2056989025005468
引用次数: 0
Abstract
The coordination complex [Zn(phen)(MBT)2], which exhibits a distorted tetrahedral geometry, was synthesized. Single-crystal X-ray analysis and Hirshfeld surface analysis revealed the presence of several intermolecular C—H⋯N and C—H⋯π interactions.
The coordination complex [Zn(C7H4NS2)2(C12H8N2)] or [Zn(MBT)2(phen)], was synthesized using ethanol solutions of Zn(CH3COO)2·2H2O, 1,10-phenanthroline (phen) and 2-mercaptobenzothiazole (MBTH-neutral). Single-crystal X-ray diffraction analysis revealed that the zinc atom resides on a crystallographic twofold axis within the asymmetric unit. In the complex, the zinc atom coordinates two 2-mercaptobenzothiazolate (MBT; anionic form of MBTH) ligands in a monodentate fashion through their sulfur atoms, while phenanthroline acts as a bidentate ligand, chelating the zinc center. Further structural analysis, including Hirshfeld surface and two-dimensional fingerprint plot studies, indicated the presence of multiple intermolecular interactions, particularly C—H⋯N and C—H⋯π interactions, contributing to the cohesion and packing of the crystal structure.
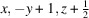
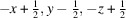
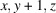
双-(苯并噻唑-2-硫醇-κ s)(1,10-phen-anthroline-κ 2n,N')锌的合成及晶体结构分析
以Zn(CH3COO)2·2H2O、1,10-菲罗啉(phenen)和2-巯基苯并噻唑(MBTH-neutral)为溶剂合成配合物[Zn(C7H4NS2)2(C12H8N2)]或[Zn(MBT)2(phen)]。单晶x射线衍射分析表明,锌原子位于不对称单元内的双轴上。在配合物中,锌原子配位两个2-巯基苯并噻唑酸盐(MBT;阴离子形式的MBTH)配体以单齿方式通过它们的硫原子,而菲罗啉作为双齿配体,螯合锌中心。进一步的结构分析,包括Hirshfeld表面和二维指纹图研究,表明存在多种分子间相互作用,特别是C-H⋯N和C-H⋯π相互作用,有助于晶体结构的内聚和堆积。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Acta Crystallographica Section E: Crystallographic Communications
Chemistry-Chemistry (all)
CiteScore
1.90
自引率
0.00%
发文量
351
审稿时长
3 weeks
期刊介绍:
Acta Crystallographica Section E: Crystallographic Communications is the IUCr''s open-access structural communications journal. It provides a fast, simple and easily accessible publication mechanism for crystal structure determinations of inorganic, metal-organic and organic compounds. The electronic submission, validation, refereeing and publication facilities of the journal ensure rapid and high-quality publication of fully validated structures. The primary article category is Research Communications; these are peer-reviewed articles describing one or more structure determinations with appropriate discussion of the science.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


