Synthesis of 6-ethoxyphenyl 4-fluorobenzenesulfonate-tagged thiosemicarbazones as carbonic anhydrase inhibitors: In-vitro and in silico approach
IF 3
3区 医学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
In this study, a series of 6-ethoxyphenyl-4-fluorobenzenesulphonate-based thiosemicarbazones (5a–w) were synthesized via a two-step process and structurally characterized by 1H NMR and 13C NMR spectroscopy. Their inhibitory activities against human carbonic anhydrase isoforms I and II (hCA I and hCA II) were evaluated, revealing potent inhibition at low nanomolar concentrations with IC50 values ranging from 56.36 to 230.17 nM for hCA I and 30.66 to 175.45 nM for hCA II. Compounds 5a, 5g, and 5n exhibited the highest enzyme inhibition, with 5a identified as the most potent in vitro inhibitor for both isoforms. Molecular docking studies and MM-GBSA binding free energy calculations demonstrated that compound 5n displayed the strongest binding affinity toward hCA I, stabilized by key interactions including π-π stacking, hydrogen bonds, and coordination to the catalytic zinc ion. Molecular dynamics simulations over 100 ns confirmed the stability and dynamic adaptability of the 5n–hCA I and 5g–hCA II complexes, preserving critical interactions essential for binding. Validation of the docking protocol yielded RMSD values below 2.0 Å, supporting the reliability of the computational approach. Overall, these findings highlight compounds 5n and 5g as promising lead molecules for selective inhibition of hCA I and hCA II, with potential applications in the treatment of carbonic anhydrase-related disorders.
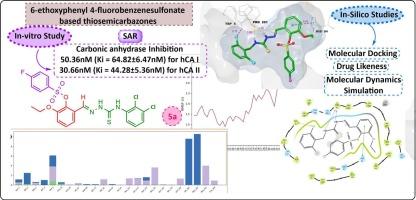
作为碳酸酐酶抑制剂的6-乙氧基苯基4-氟苯磺酸标记的硫代氨基脲的合成:体外和硅法
本研究采用两步法合成了一系列6-乙氧基苯基-4-氟苯磺酸基硫代氨基脲类化合物(5a-w),并通过1H NMR和13C NMR对其进行了结构表征。对其对人类碳酸酐酶I和II型(hCA I和hCA II)的抑制活性进行了评价,发现在低纳摩尔浓度下,hCA I的IC50值为56.36 ~ 230.17 nM, hCA II的IC50值为30.66 ~ 175.45 nM。化合物5a、5g和5n表现出最高的酶抑制作用,其中5a被认为是对这两种异构体最有效的体外抑制剂。分子对接研究和MM-GBSA结合自由能计算表明,化合物5n对hCA I的结合亲和力最强,通过π-π堆叠、氢键和与催化锌离子的配位等关键相互作用稳定。超过100 ns的分子动力学模拟证实了5n-hCA I和5g-hCA II配合物的稳定性和动态适应性,保留了结合所必需的关键相互作用。对接协议验证的RMSD值低于2.0 Å,支持计算方法的可靠性。总的来说,这些发现突出了化合物5n和5g作为选择性抑制hCA I和hCA II的有希望的先导分子,在治疗碳酸酐酶相关疾病方面具有潜在的应用前景。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Bioorganic & Medicinal Chemistry
医学-生化与分子生物学
CiteScore
6.80
自引率
2.90%
发文量
413
审稿时长
17 days
期刊介绍:
Bioorganic & Medicinal Chemistry provides an international forum for the publication of full original research papers and critical reviews on molecular interactions in key biological targets such as receptors, channels, enzymes, nucleotides, lipids and saccharides.
The aim of the journal is to promote a better understanding at the molecular level of life processes, and living organisms, as well as the interaction of these with chemical agents. A special feature will be that colour illustrations will be reproduced at no charge to the author, provided that the Editor agrees that colour is essential to the information content of the illustration in question.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


