Reciprocal c-Abl-GPx1 regulation controls CA1 neuronal viability to oxidative stress via ERK1/2-DRP1-mediated mitochondrial dynamics
IF 4.6
2区 医学
Q1 NEUROSCIENCES
引用次数: 0
Abstract
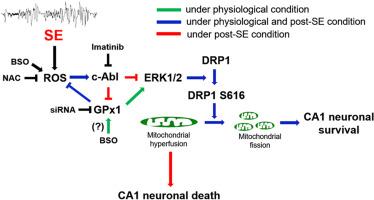
Abelson murine leukemia viral oncogene homolog 1 (c-Abl, also known as ABL1) is a potent selenium-independent regulator of expression and activity of glutathione peroxidase-1 (GPx1) and extracellular signal-regulated kinase 1/2 (ERK1/2). Since GPx1-ERK1/2 pathway modulates dynamin-related protein 1 (DRP1) serine (S) 616 phosphorylation, we investigated whether c-Abl participates in GPx1-ERK1/2 interaction and DRP1-mediated mitochondrial dynamics in CA1 neurons in response to oxidative stress induced by L-buthionine sulfoximine (BSO, an oxidative stress inducer) and status epilepticus (SE). In the present study, BSO enhanced c-Abl tyrosine (Y) 245 phosphorylation, ERK1/2 activity and GPx1 upregulation in the CA1 region under physiological condition. Imatinib (a c-Abl inhibitor) ameliorated BSO-induced c-Abl Y245, but elicited further ERK1/2 phosphorylation without affecting GPx1 expression. GPx1 knockdown enhanced BSO-induced c-Abl Y245 phosphorylation, but decreased ERK1/2 activity. BSO also facilitated mitochondrial fission in CA1 neurons by augmenting DRP1 expression and its S616 phosphorylation in the CA1 region, which were diminished by GPx1 knockdown and U0126 (an ERK1/2 inhibitor), but reinforced by imatinib. SE increased c-Abl Y245 phosphorylation and mitochondrial length in CA1 neurons, accompanied by reduced GPx1 expression and ERK1/2 phosphorylation. Imatinib and N-acetylcysteine (NAC, an antioxidant) attenuated these post-SE events and CA1 neuronal death. However, GPx1 knockdown deteriorated SE-induced CA1 neuronal degeneration accompanied by augmenting c-Abl Y245 phosphorylation and mitochondrial elongation in CA1 neurons. These findings indicate that the impaired reciprocal regulation between c-Abl and GPx1 may cause CA1 neuronal degeneration in response to oxidative stress by abrogating ERK1/2-DRP1-mediated mitochondrial fission.
通过erk1 /2- drp1介导的线粒体动力学,互惠的c-Abl-GPx1调节CA1神经元对氧化应激的活力
Abelson小鼠白血病病毒癌基因同源物1 (c-Abl,也称为ABL1)是谷胱甘肽过氧化物酶1 (GPx1)和细胞外信号调节激酶1/2 (ERK1/2)表达和活性的有效硒独立调节剂。由于GPx1-ERK1/2通路调节动力蛋白相关蛋白1 (DRP1)丝氨酸(S) 616磷酸化,我们研究了c-Abl是否参与GPx1-ERK1/2相互作用和DRP1介导的CA1神经元线粒体动力学,以响应l -丁硫氨酸亚砜(BSO,一种氧化应激诱导剂)和癫痫持续状态(SE)诱导的氧化应激。在本研究中,BSO增强了生理条件下c-Abl酪氨酸(Y) 245磷酸化、ERK1/2活性和CA1区GPx1上调。伊马替尼(一种c-Abl抑制剂)改善了bso诱导的c-Abl Y245,但在不影响GPx1表达的情况下引发了ERK1/2的进一步磷酸化。GPx1敲低增强了bso诱导的c-Abl Y245磷酸化,但降低了ERK1/2活性。BSO还通过增加DRP1的表达及其在CA1区域的S616磷酸化促进了CA1神经元的线粒体分裂,GPx1敲低和U0126 (ERK1/2抑制剂)减少了这些磷酸化,但伊马替尼增强了这些磷酸化。SE增加CA1神经元c-Abl Y245磷酸化和线粒体长度,同时GPx1表达和ERK1/2磷酸化减少。伊马替尼和n -乙酰半胱氨酸(NAC,一种抗氧化剂)减轻了这些se后事件和CA1神经元死亡。然而,GPx1敲低恶化了se诱导的CA1神经元变性,同时CA1神经元中c-Abl Y245磷酸化和线粒体伸长增加。这些发现表明,c-Abl和GPx1之间的相互调节受损可能通过取消erk1 /2- drp1介导的线粒体裂变而导致CA1神经元变性,以响应氧化应激。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Neuropharmacology
医学-神经科学
CiteScore
10.00
自引率
4.30%
发文量
288
审稿时长
45 days
期刊介绍:
Neuropharmacology publishes high quality, original research and review articles within the discipline of neuroscience, especially articles with a neuropharmacological component. However, papers within any area of neuroscience will be considered. The journal does not usually accept clinical research, although preclinical neuropharmacological studies in humans may be considered. The journal only considers submissions in which the chemical structures and compositions of experimental agents are readily available in the literature or disclosed by the authors in the submitted manuscript. Only in exceptional circumstances will natural products be considered, and then only if the preparation is well defined by scientific means. Neuropharmacology publishes articles of any length (original research and reviews).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


