Mechanistic study of pexidartinib-induced toxicity in human hepatic cells
IF 5.4
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Pexidartinib, a tyrosine kinase inhibitor, was approved by the U.S. Food and Drug Administration in 2019 for treating adult patients with symptomatic tenosynovial giant cell tumors. Because of its hepatotoxicity risks, pexidartinib received a boxed warning; however, mechanistic studies on this hepatotoxicity remain limited. In this study, we demonstrate that pexidartinib decreases cell viability in primary human hepatocytes and hepatic HepG2 cells. A 24-h treatment with pexidartinib led to apoptosis in HepG2 cells, as evidenced by increased caspase 3/7 activity and the induction of cleaved PARP and γ-H2A.X. Pexidartinib-induced endoplasmic reticulum (ER) stress was observed at early time points of 2 h and 5 h. The ER stress inhibitor 4-phenylbutyrate (4-PBA) partially attenuated pexidartinib-induced cytotoxicity. Additionally, mitochondrial dysfunction caused by pexidartinib was indicated by a strong inhibition of mitochondrial respiratory complexes II, IV, and V, a loss of mitochondrial potential, and greater toxicity in galactose media compared to glucose media. Pexidartinib also induced autophagy, as demonstrated by the formation of autophagosomes and autophagic flux. We investigated the role of cytochrome P450 (CYP)-mediated metabolism in pexidartinib-induced cytotoxicity by using HepG2 cell lines engineered to express 14 individual CYPs. The results indicated that CYP1A1, 2C9, 3A4, and 3A5 were involved in pexidartinib metabolism. Inhibition of CYP1A1, 2C9, and 3A4/5 significantly increased pexidartinib-induced cytotoxicity in primary human hepatocytes. Collectively, these data suggest that pexidartinib induced apoptosis, ER stress, mitochondria dysfunction, and autophagy in hepatic cells, and CYPs-mediated metabolism played a protective role in reducing pexidartinib toxicity.
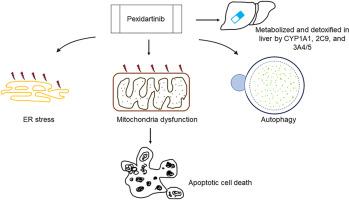
培西达替尼对人肝细胞毒性的机制研究。
佩西达替尼是一种酪氨酸激酶抑制剂,于2019年被美国食品和药物管理局批准用于治疗成年症状性腱鞘巨细胞瘤患者。由于其肝毒性风险,培西达替尼收到了黑框警告;然而,关于这种肝毒性的机制研究仍然有限。在这项研究中,我们证明了培西达替尼降低了原代人肝细胞和肝HepG2细胞的细胞活力。培西达替尼24小时可导致HepG2细胞凋亡,caspase 3/7活性增加,裂解PARP和γ-H2A.X表达增加。培西达替尼诱导的内质网(ER)应激发生在2 h和5 h的早期时间点。内质网应激抑制剂4-苯基丁酸(4-PBA)部分减弱了培西达替尼诱导的细胞毒性。此外,培西达替尼引起的线粒体功能障碍表现为对线粒体呼吸复合物II、IV和V的强烈抑制,线粒体电位的丧失,以及在半乳糖培养基中比在葡萄糖培养基中毒性更大。培西达替尼也诱导自噬,自噬体的形成和自噬通量证明了这一点。我们利用表达14个CYP的HepG2细胞系,研究了细胞色素P450 (CYP)介导的代谢在培西达替尼诱导的细胞毒性中的作用。结果表明CYP1A1、2C9、3A4和3A5参与培西达替尼代谢。抑制CYP1A1、2C9和3A4/5显著增加培西达替尼诱导的人原代肝细胞的细胞毒性。综上所述,这些数据表明,培西达替尼诱导肝细胞凋亡、内质网应激、线粒体功能障碍和自噬,cyps介导的代谢在降低培西达替尼毒性中起保护作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.70
自引率
3.90%
发文量
410
审稿时长
36 days
期刊介绍:
Chemico-Biological Interactions publishes research reports and review articles that examine the molecular, cellular, and/or biochemical basis of toxicologically relevant outcomes. Special emphasis is placed on toxicological mechanisms associated with interactions between chemicals and biological systems. Outcomes may include all traditional endpoints caused by synthetic or naturally occurring chemicals, both in vivo and in vitro. Endpoints of interest include, but are not limited to carcinogenesis, mutagenesis, respiratory toxicology, neurotoxicology, reproductive and developmental toxicology, and immunotoxicology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


