SLC34A2 inhibits tumorigenesis and progression via upregulating LRRK2/TTF-1/SELENBP1 axis in lung adenocarcinoma
IF 5
3区 医学
Q1 BIOTECHNOLOGY & APPLIED MICROBIOLOGY
引用次数: 0
Abstract
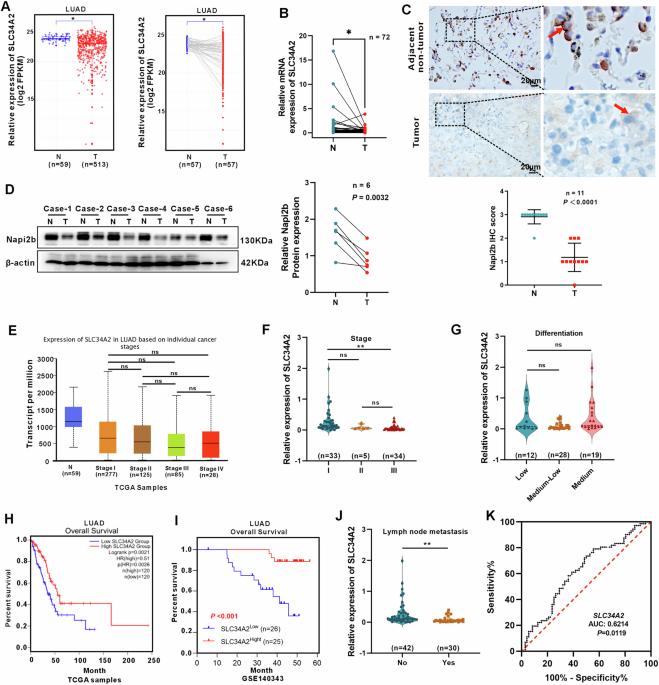
Alveolar type II epithelial (AT2) cells have the properties of stem cells, abnormal AT2 cells serve as one of the original cells in lung adenocarcinoma (LUAD). However, the abnormal genes expression of AT2 cells during their malignant transformation into LUAD cells remain poorly understood. Importantly, SLC34A2 is a specific gene in AT2 cells of the lung. Our previous researches have reported that overexpression of SLC34A2 significantly inhibited proliferation, migration and invasion of LUAD cells. But, the underlying mechanisms of SLC34A2 in LUAD are largely unknown until now. Here, the present study discovered that the protein expression of Napi2b (SLC34A2), SELENBP1, TTF-1 and LRRK2 were all located in human AT2 cells of adjacent non-tumor tissues. However, the expression level of SLC34A2, SELENBP1, TTF-1 and LRRK2 were significantly decreased in LUAD tissues, and the expression of SLC34A2 was obviously positive correlation with the expression of SELENBP1, TTF-1 and LRRK2, respectively. Mechanistically, our study elucidated that overexpression of SLC34A2 could inhibit the activation of MEK/ERK signaling pathway through up-regulating the expression of LRRK2, and subsequently suppressed the expression of p-TTF-1(Ser327), which upregulated the expression of SELENBP1 by enhancing TTF-1 transcriptional activity. Ultimately, overexpression of SLC34A2 depressed the activation of PI3K/AKT/mTOR signaling pathway via up-regulating the expression of SELENBP1, which significantly inhibited the malignant characteristics of LUAD. In summary, our current research revealed a novel SLC34A2/LRRK2/TTF-1/SELENBP1 axis and its involvement in inhibiting the malignant characteristics of LUAD cells for the first time, which made contribution to further exploring the clinical application of SLC34A2. Furthermore, it also might offer novel insights into understanding how AT2 cells undergo malignant transformation into LUAD cells in the future.
SLC34A2通过上调LRRK2/TTF-1/SELENBP1轴在肺腺癌中抑制肿瘤的发生和进展。
肺泡II型上皮细胞(Alveolar type II epithelial, AT2)具有干细胞的特性,异常的AT2细胞是肺腺癌(LUAD)的原发细胞之一。然而,AT2细胞在恶性转化为LUAD细胞过程中基因的异常表达尚不清楚。重要的是,SLC34A2是肺AT2细胞中的特异性基因。我们前期研究报道过表达SLC34A2可显著抑制LUAD细胞的增殖、迁移和侵袭。但是,到目前为止,SLC34A2在LUAD中的潜在机制在很大程度上是未知的。本研究发现,Napi2b (SLC34A2)、SELENBP1、TTF-1和LRRK2的蛋白表达均位于邻近非肿瘤组织的人AT2细胞中。而在LUAD组织中,SLC34A2、SELENBP1、TTF-1和LRRK2的表达水平均显著降低,且SLC34A2的表达分别与SELENBP1、TTF-1和LRRK2的表达呈明显正相关。机制上,我们的研究阐明了SLC34A2过表达可以通过上调LRRK2的表达抑制MEK/ERK信号通路的激活,进而抑制p-TTF-1(Ser327)的表达,从而通过增强TTF-1的转录活性上调SELENBP1的表达。最终,SLC34A2过表达通过上调SELENBP1的表达,抑制PI3K/AKT/mTOR信号通路的激活,从而显著抑制LUAD的恶性特征。综上所述,本研究首次发现新的SLC34A2/LRRK2/TTF-1/SELENBP1轴及其参与抑制LUAD细胞恶性特征,为进一步探索SLC34A2的临床应用做出了贡献。此外,它也可能在未来为理解AT2细胞如何恶性转化为LUAD细胞提供新的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cancer gene therapy
医学-生物工程与应用微生物
CiteScore
10.20
自引率
0.00%
发文量
150
审稿时长
4-8 weeks
期刊介绍:
Cancer Gene Therapy is the essential gene and cellular therapy resource for cancer researchers and clinicians, keeping readers up to date with the latest developments in gene and cellular therapies for cancer. The journal publishes original laboratory and clinical research papers, case reports and review articles. Publication topics include RNAi approaches, drug resistance, hematopoietic progenitor cell gene transfer, cancer stem cells, cellular therapies, homologous recombination, ribozyme technology, antisense technology, tumor immunotherapy and tumor suppressors, translational research, cancer therapy, gene delivery systems (viral and non-viral), anti-gene therapy (antisense, siRNA & ribozymes), apoptosis; mechanisms and therapies, vaccine development, immunology and immunotherapy, DNA synthesis and repair.
Cancer Gene Therapy publishes the results of laboratory investigations, preclinical studies, and clinical trials in the field of gene transfer/gene therapy and cellular therapies as applied to cancer research. Types of articles published include original research articles; case reports; brief communications; review articles in the main fields of drug resistance/sensitivity, gene therapy, cellular therapy, tumor suppressor and anti-oncogene therapy, cytokine/tumor immunotherapy, etc.; industry perspectives; and letters to the editor.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


