Jagandeep S. Saraya, Derek K. O'Flaherty
{"title":"An Aqueous-Compatible On-Column Approach for the Conjugation of Nucleic Acids Using Amino Modifiers","authors":"Jagandeep S. Saraya, Derek K. O'Flaherty","doi":"10.1002/cpz1.70169","DOIUrl":null,"url":null,"abstract":"<p>Nucleic acid conjugation has emerged as a powerful strategy for enhancing the chemical and biological versatility of synthetic oligonucleotides. Solid-support synthesis of oligonucleotides provides an avenue for nucleic acid conjugation, so long as the method is inherently compatible with the synthesis cycle. Many methods exist, post-synthetically (i.e., after strand extension via the DNA synthesizer), to add ligands to the strand while it is still bound to the solid support. These, however, tend to require stringent reaction conditions (i.e., anhydrous, degassed solvents, special apparatus), making them sometimes impractical for routine use. This article describes a streamlined aqueous-compatible on-column conjugation strategy for preparing nucleic acids containing site-specific chemical modifications. This method utilizes commercially available or easily synthesized monophosphate or carboxylate-containing ligands and solid-phase synthesized oligonucleotides containing amino-modified termini. Coupling is enabled by 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) and <i>N</i>-methylimidazole (<i>N</i>-MeIm) chemistry in buffered aqueous-organic mixtures. The resulting conjugates are processed using typical deprotection and solid-support cleavage protocols, purified by standard techniques such as strong anion exchange high-performance liquid chromatography (SAX-HPLC), and are characterized by mass spectrometry (MS). © 2025 The Author(s). Current Protocols published by Wiley Periodicals LLC.</p><p><b>Basic Protocol 1</b>: Synthesis of zidovudine 5′-<i>O</i>-monophosphate</p><p><b>Basic Protocol 2</b>: On-column phosphoramidate-mediated conjugation of 5′-<i>O</i>-phosphorylated AZT to 5′-amino DNA</p><p><b>Basic Protocol 3</b>: On-column amide-mediated conjugation of Fmoc-protected glycine to 5′-amino modified DNA</p><p><b>Basic Protocol 4</b>: On-column amide-mediated conjugation of potassium benzoate to 5′-amino modified RNA</p>","PeriodicalId":93970,"journal":{"name":"Current protocols","volume":"5 7","pages":""},"PeriodicalIF":2.2000,"publicationDate":"2025-07-04","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://onlinelibrary.wiley.com/doi/epdf/10.1002/cpz1.70169","citationCount":"0","resultStr":null,"platform":"Semanticscholar","paperid":null,"PeriodicalName":"Current protocols","FirstCategoryId":"1085","ListUrlMain":"https://currentprotocols.onlinelibrary.wiley.com/doi/10.1002/cpz1.70169","RegionNum":0,"RegionCategory":null,"ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"","JCRName":"","Score":null,"Total":0}
引用次数: 0
Abstract
Nucleic acid conjugation has emerged as a powerful strategy for enhancing the chemical and biological versatility of synthetic oligonucleotides. Solid-support synthesis of oligonucleotides provides an avenue for nucleic acid conjugation, so long as the method is inherently compatible with the synthesis cycle. Many methods exist, post-synthetically (i.e., after strand extension via the DNA synthesizer), to add ligands to the strand while it is still bound to the solid support. These, however, tend to require stringent reaction conditions (i.e., anhydrous, degassed solvents, special apparatus), making them sometimes impractical for routine use. This article describes a streamlined aqueous-compatible on-column conjugation strategy for preparing nucleic acids containing site-specific chemical modifications. This method utilizes commercially available or easily synthesized monophosphate or carboxylate-containing ligands and solid-phase synthesized oligonucleotides containing amino-modified termini. Coupling is enabled by 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) and N-methylimidazole (N-MeIm) chemistry in buffered aqueous-organic mixtures. The resulting conjugates are processed using typical deprotection and solid-support cleavage protocols, purified by standard techniques such as strong anion exchange high-performance liquid chromatography (SAX-HPLC), and are characterized by mass spectrometry (MS). © 2025 The Author(s). Current Protocols published by Wiley Periodicals LLC.
Basic Protocol 1: Synthesis of zidovudine 5′-O-monophosphate
Basic Protocol 2: On-column phosphoramidate-mediated conjugation of 5′-O-phosphorylated AZT to 5′-amino DNA
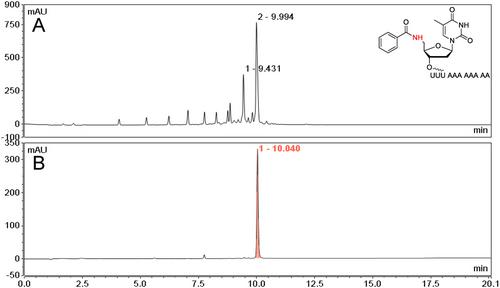
Basic Protocol 3: On-column amide-mediated conjugation of Fmoc-protected glycine to 5′-amino modified DNA
Basic Protocol 4: On-column amide-mediated conjugation of potassium benzoate to 5′-amino modified RNA




用氨基修饰剂偶联核酸的水相容柱上方法
核酸偶联已成为增强合成寡核苷酸的化学和生物多功能性的一种强有力的策略。固体载体合成寡核苷酸为核酸偶联提供了一条途径,只要该方法与合成周期内在兼容。存在许多方法,合成后(即通过DNA合成器延长链后),在链仍然与固体载体结合时向链添加配体。然而,这些往往需要严格的反应条件(即,无水,脱气溶剂,特殊设备),使它们有时不适合常规使用。本文描述了一种流线型的水相容柱上偶联策略,用于制备含有位点特异性化学修饰的核酸。本方法利用市售或容易合成的含单磷酸盐或羧酸的配体和固相合成的含氨基修饰末端的寡核苷酸。偶联是通过1-乙基-3-(3-二甲氨基丙基)碳二亚胺(EDC)和n -甲基咪唑(N-MeIm)在缓冲水-有机混合物中的化学作用实现的。所得的共轭物采用典型的脱保护和固体支撑裂解工艺进行处理,采用强阴离子交换高效液相色谱(SAX-HPLC)等标准技术进行纯化,并用质谱(MS)进行表征。©2025作者。Wiley期刊有限责任公司发表的当前方案:基本方案1:齐多夫定5 ' - o -单磷酸的合成;基本方案2:5 ' - o -磷酸化AZT的柱上磷酸介导偶联到5 ' -氨基dna;基本方案3:fmoc保护的甘氨酸的柱上酰胺介导偶联到5 ' -氨基修饰的dna;基本方案4:苯甲酸钾的柱上酰胺介导偶联到5 ' -氨基修饰的RNA
本文章由计算机程序翻译,如有差异,请以英文原文为准。






 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


