The role of extracellular vesicles in chronic lung allograft dysfunction and response to extracorporeal photopheresis
引用次数: 0
Abstract
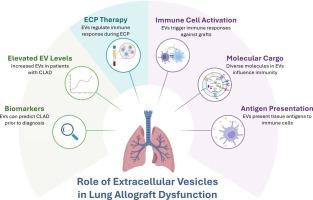
Current research highlights the growing role of extracellular vesicles (EV) in mechanisms of lung allograft dysfunction. In particular, EVs are involved in antigen presentation, where they are released from lung allografts and express tissue associated antigens which are recognized by recipient immune cells, thereby triggering an immune response against the transplanted lung. In the context of chronic rejection, patients with chronic lung allograft dysfunction (CLAD) demonstrate elevated levels of EVs, which contain diverse molecular cargo that can influence the alloimmune response. This highlights the potential of EVs as translatable biomarkers for the early detection, prediction, or diagnosis of lung allograft dysfunction. The mechanisms by which EVs contribute to this process may include immune cell activation, epithelial-to-mesenchymal transition, and disruption of angiogenesis. Furthermore, their immunomodulatory potential is evident by their emerging involvement in regulating the immune response during extracorporeal photopheresis (ECP) therapy following lung transplantation, where they contribute to the balance of immunoregulatory and autoimmune responses within a highly interwoven network. While ECP shows promise for broader or earlier use in solid organ transplantation, its application is limited by a lack of mechanistic understanding. This review summarizes the role of EVs in development of lung allograft dysfunction, their involvement in immunomodulation, and the current literature exploring their potential role in the mechanisms of ECP therapy.
细胞外囊泡在慢性同种异体肺移植功能障碍中的作用和对体外光再生的反应
目前的研究强调细胞外囊泡(EV)在肺移植功能障碍机制中的作用越来越大。特别是,ev参与抗原呈递,它们从肺同种异体移植物中释放出来并表达组织相关抗原,这些抗原被受体免疫细胞识别,从而引发针对移植肺的免疫反应。在慢性排斥反应的背景下,慢性肺同种异体移植物功能障碍(chronic lung allograft dysfunction, CLAD)患者表现出EVs水平升高,其中含有多种影响同种异体免疫反应的分子货物。这凸显了ev作为早期检测、预测或诊断同种异体肺移植功能障碍的可翻译生物标志物的潜力。EVs参与这一过程的机制可能包括免疫细胞活化、上皮细胞向间质细胞转化和血管生成的破坏。此外,它们的免疫调节潜力是显而易见的,因为它们在肺移植后体外光造血(ECP)治疗期间参与调节免疫反应,在一个高度交织的网络中,它们有助于免疫调节和自身免疫反应的平衡。虽然ECP在实体器官移植中有更广泛或更早的应用前景,但由于缺乏对其机制的理解,其应用受到限制。本文综述了EVs在同种异体移植肺功能障碍发展中的作用,EVs在免疫调节中的作用,以及目前探讨EVs在ECP治疗机制中的潜在作用的文献。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


