Design, synthesis and evaluation of vanillin derivatives as dual-target inhibitors for the treatment of Alzheimer's disease
IF 3
3区 医学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
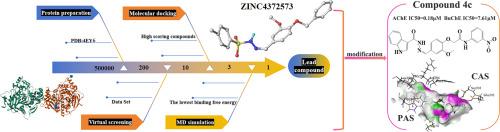
The purpose of this study is to develop more effective therapeutic agents to slow or prevent Alzheimer's progression. A lead compound ZINC4372573 was identified by using molecular docking and molecular dynamics simulation techniques. A series of novel vanillin derivatives were designed and synthesized as dual inhibitors of acetylcholinesterase (AChE) and butyrylcholinesterase (BuChE). The in vitro assay results show that compound 4c exhibits the most potent inhibitory activity against both AChE and BuChE, with IC50 values of 0.18 μM and 7.61 μM, respectively. This performance is superior to the positive control drug galantamine (AChE IC50 = 3.65 μM; BuChE IC50 = 15.29 μM). Molecular docking study reveals that the good activity of 4c may be attributed to the preferable docking mode, robust intermolecular interactions (including π-π stacking and hydrogen bonding), and the superior binding properties of the indole ring. Cytotoxicity test for compound 4c was further performed by CCK-8 method, with results indicating a favorable safety profile. In addition, antioxidant test for 4c reveals its notable antioxidant activity. These findings suggest that 4c holds potential as a promising dual AChE/BuChE inhibitor for the development of novel therapeutic agents targeting Alzheimer's disease. Subsequent investigations will prioritize comprehensive evaluation of in vivo therapeutic efficacy and pharmacokinetic characterization, thereby facilitating translational development toward clinical applications.
香兰素衍生物作为治疗阿尔茨海默病双靶点抑制剂的设计、合成和评价
这项研究的目的是开发更有效的治疗药物来减缓或预防阿尔茨海默病的进展。采用分子对接和分子动力学模拟技术,鉴定了一种先导化合物ZINC4372573。设计并合成了一系列新型香兰素衍生物,作为乙酰胆碱酯酶(AChE)和丁基胆碱酯酶(BuChE)的双重抑制剂。体外实验结果表明,化合物4c对AChE和BuChE的抑制活性最强,IC50值分别为0.18 μM和7.61 μM。该性能优于阳性对照药物加兰他明(AChE IC50 = 3.65 μM;BuChE IC50 = 15.29 μM)。分子对接研究表明,4c具有良好的对接方式、良好的分子间相互作用(包括π-π堆叠和氢键)以及吲哚环良好的结合性能可能是其良好活性的主要原因。进一步采用CCK-8法对化合物4c进行细胞毒性试验,结果表明化合物4c具有良好的安全性。此外,对4c进行了抗氧化试验,结果表明其抗氧化活性显著。这些发现表明,4c作为AChE/BuChE双重抑制剂具有开发针对阿尔茨海默病的新型治疗药物的潜力。后续研究将优先考虑体内治疗效果和药代动力学特征的综合评价,从而促进转化开发的临床应用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Bioorganic & Medicinal Chemistry
医学-生化与分子生物学
CiteScore
6.80
自引率
2.90%
发文量
413
审稿时长
17 days
期刊介绍:
Bioorganic & Medicinal Chemistry provides an international forum for the publication of full original research papers and critical reviews on molecular interactions in key biological targets such as receptors, channels, enzymes, nucleotides, lipids and saccharides.
The aim of the journal is to promote a better understanding at the molecular level of life processes, and living organisms, as well as the interaction of these with chemical agents. A special feature will be that colour illustrations will be reproduced at no charge to the author, provided that the Editor agrees that colour is essential to the information content of the illustration in question.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


