Cycloaddition Reaction of N-Homoallylacetohydrazides with O-Benzoylhydroxylamines under Copper Catalysis: Access to Tetrahydropyridazine Skeletons
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
We present a practical copper-catalyzed strategy for the amination and cyclization of unactivated alkenes. The transformation employs O-benzoylhydroxylamines as alkylamine precursors to initiate a radical cascade process. This protocol enables the construction of a diverse array of tetrahydropyridazine scaffolds by reacting N-homoallylacetohydrazides with O-benzoylhydroxylamines, ultimately affording a total of 25 structurally varied derivatives.
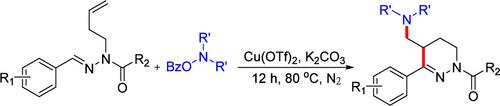
铜催化下n -纯烯丙基乙酰肼与邻苯甲酰羟胺的环加成反应:获得四氢吡啶骨架。
我们提出了一个实用的铜催化策略的胺化和环化的非活化烯烃。该转化采用邻苯甲酰羟胺作为烷基胺前体来启动自由基级联过程。该方案通过n -均烯丙基乙酰肼与o -苯甲酰羟胺反应,可以构建各种各样的四氢吡嗪支架,最终提供总共25种结构不同的衍生物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


