Design, synthesis, anticancer activity, and in silico computational studies of new imidazolone-based derivatives with potential multi-target kinase inhibitory activity
IF 3
3区 医学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
A novel class of 5-(3,5-dimethoxybenzylidene)-2-thioxoimidazolidin-4-one-based derivatives, linked to various alkyl and aryl substituents 1a-d and 2a-h, was designed and synthesized as promising candidates for anti-colon cancer therapy with multi-targeting kinase suppression activity. The antiproliferative effect of the new compounds was assessed against HT-29 using the MTT assay. The congeners 1c and 2h demonstrated the most potent suppressive effects, with IC50 values 1.828 and 2.197 μg/mL, respectively. The latter derivatives were evaluated as multi-kinase inhibitors against VEGFR-2, c-Met, and PIM-1, exhibiting promising activity with IC50 values ranging from 0.081 ± 0.003 to 0.433 ± 0.017 μg/mL. Moreover, 2h induced an apoptotic effect and cell cycle arrest at G0/G1 of the mitotic cycle in HT-29 cells. Furthermore, 2h upregulated the oncogenic parameters, including caspase-3, caspase-9, and the Bax/Bcl-2 ratio. The docking results showed that compounds 1c, 2h, and 2e had strong binding energies and effectively interacted with the active sites of the VEGFR-2, c-Met, and PIM-1 receptors. According to the in-silico ADMET analysis the new compounds are anticipated to exhibit promising oral bioavailability, desirable drug-like qualities, and minimal toxicity risks. Molecular dynamics (MD) simulations indicated that 2h interacts consistently with the c-MET, PIM-1, and VEGFR-2 receptors. These results reinforce the potential of these compounds as candidates for further drug development.
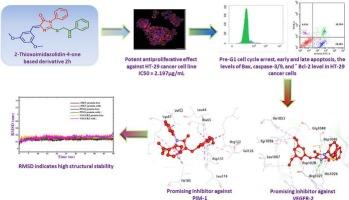
具有潜在多靶点激酶抑制活性的新型咪唑酮衍生物的设计、合成、抗癌活性和计算机计算研究
设计并合成了一类新的5-(3,5-二甲氧基苄基)-2-硫氧基咪唑烷-4- 1基衍生物,它们与不同的烷基和芳基取代基1a-d和2a-h连接,具有多靶点激酶抑制活性,是抗结肠癌治疗的有希望的候选药物。采用MTT法评估新化合物对HT-29的抗增殖作用。同源基因1c和2h的抑制作用最强,IC50值分别为1.828和2.197 μg/mL。后一种衍生物被评价为VEGFR-2、c-Met和PIM-1的多激酶抑制剂,具有良好的活性,IC50值为0.081±0.003 ~ 0.433±0.017 μg/mL。此外,2h诱导HT-29细胞的凋亡效应和细胞周期阻滞在有丝分裂周期的G0/G1。此外,2h上调了致癌参数,包括caspase-3、caspase-9和Bax/Bcl-2比值。对接结果表明,化合物1c、2h和2e具有较强的结合能,能与VEGFR-2、c-Met和PIM-1受体的活性位点有效相互作用。根据计算机ADMET分析,新化合物有望表现出有希望的口服生物利用度,理想的药物样质量和最小的毒性风险。分子动力学(MD)模拟表明,2h与c-MET、PIM-1和VEGFR-2受体相互作用一致。这些结果加强了这些化合物作为进一步药物开发候选物的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Bioorganic & Medicinal Chemistry
医学-生化与分子生物学
CiteScore
6.80
自引率
2.90%
发文量
413
审稿时长
17 days
期刊介绍:
Bioorganic & Medicinal Chemistry provides an international forum for the publication of full original research papers and critical reviews on molecular interactions in key biological targets such as receptors, channels, enzymes, nucleotides, lipids and saccharides.
The aim of the journal is to promote a better understanding at the molecular level of life processes, and living organisms, as well as the interaction of these with chemical agents. A special feature will be that colour illustrations will be reproduced at no charge to the author, provided that the Editor agrees that colour is essential to the information content of the illustration in question.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


