ASIC1a-associated mechanical hypersensitivity in the GlaKO Fabry disease mouse model
Q2 Medicine
引用次数: 0
Abstract
Different lines of evidence point to a role for Acid-sensing ion channel 1 (ASIC1) in pain perception, acting as sensors in both the central nervous system and peripheral tissues. While elevated ASIC1 protein expression has been documented in various pain conditions, our study focuses on its involvement in the context of Fabry disease (FD).
Using a mouse model of FD, we observed a significant increase in ASIC1 protein expression in pain-related areas including the anterior cingulate cortex (ACC), as well as the spinal cord (SC) and dorsal root ganglia (DRG) at the lumbar, thoracic, and cervical levels. This upregulation was accompanied by increased ASIC1a mRNA levels and ERK phosphorylation. Moreover, in FD mice, ASIC1 protein expression was found to be modulated by age and sex: it was higher in female mice than in males, and increased with age in both sexes.
These findings, together with our previous work showing unaltered ASIC1a mRNA levels but microRNA-mediated regulation of ASIC1a protein in the formalin-induced acute pain model, highlight distinct mechanisms of ASIC1a regulation in FD-associated versus acute pain. Additionally, our study revealed heightened mechanical sensitivity in FD mice that could be prevented using a channel blocker, further highlighting the involvement of ASIC1a channels in pain pathways associated with Fabry disease. Our findings suggest that ASIC1a channels may serve as promising therapeutic targets for pain management in Fabry disease.
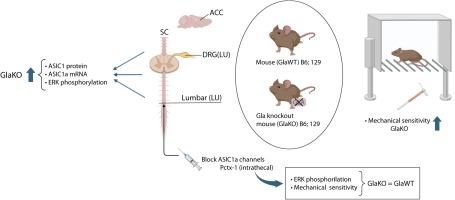
GlaKO法布里病小鼠模型中asic1a相关的机械超敏反应
不同的证据表明酸感离子通道1 (ASIC1)在疼痛感知中起作用,在中枢神经系统和外周组织中都起传感器的作用。虽然ASIC1蛋白表达升高已被记录在各种疼痛状况中,但我们的研究重点是它与法布里病(FD)的关系。通过小鼠FD模型,我们观察到ASIC1蛋白在疼痛相关区域的表达显著增加,包括前扣带皮层(ACC),以及腰椎、胸椎和颈椎水平的脊髓(SC)和背根神经节(DRG)。这种上调伴随着ASIC1a mRNA水平和ERK磷酸化的增加。此外,在FD小鼠中,发现ASIC1蛋白的表达受年龄和性别的调节:雌性小鼠的ASIC1蛋白表达高于雄性,并且随着年龄的增长,雌雄小鼠的ASIC1蛋白表达均增加。这些发现,加上我们之前的工作显示ASIC1a mRNA水平不变,但在福尔马林诱导的急性疼痛模型中,ASIC1a蛋白的microrna介导调节,突出了ASIC1a在fd相关和急性疼痛中的不同调节机制。此外,我们的研究显示,FD小鼠的机械敏感性升高可以通过通道阻滞剂来预防,进一步强调了ASIC1a通道参与与Fabry病相关的疼痛通路。我们的研究结果表明ASIC1a通道可能作为Fabry病疼痛管理的有希望的治疗靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Neurobiology of Pain
Medicine-Anesthesiology and Pain Medicine
CiteScore
4.40
自引率
0.00%
发文量
29
审稿时长
54 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


