Feasibility and acceptability of persons on long-acting cabotegravir for HIV prevention in the SEARCH Dynamic Choice HIV Prevention trial extension in rural Kenya and Uganda: a longitudinal cohort study
Abstract
Introduction
Injectable cabotegravir (CAB-LA) is highly effective for HIV prevention, but real-world implementation studies in Africa are ongoing. We assessed feasibility and acceptability among participants who used CAB-LA in the SEARCH Dynamic Choice HIV Prevention extension study in rural Uganda and Kenya.
Methods
From January 2023 to December 2024, we followed females and males who were aged ≥ 15 years, with self-assessed risk for HIV acquisition, in the intervention arm of the SEARCH Dynamic Choice HIV Prevention extension study, and received at least one CAB-LA injection during the first 48 weeks. To assess the feasibility and acceptability of CAB-LA, we designed quantitative surveys based on the Theoretical Framework for Acceptability. Surveys were administered at CAB-LA initiation, after 24 and 48 weeks of use, and discontinuation of CAB-LA.
Results
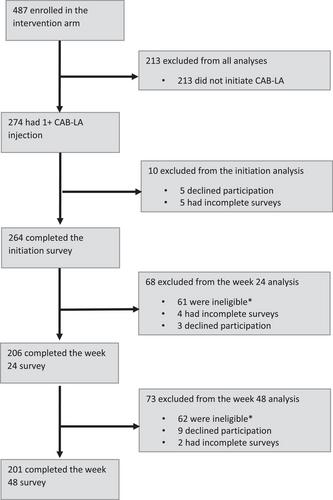
Of 487 intervention arm participants, 274 (56%) started CAB-LA (183 females; 91 males; 79 youth aged 15–24 years). Of whom, 264 completed the survey at initiation, 206 after 24 weeks on CAB-LA, 201 after 48 weeks on CAB-LA and 69 at discontinuation of CAB-LA. Most participants (65%; 171/264) reported choosing CAB-LA because it was easier to take than pills, and nearly all (99%; 261/264) had limited knowledge of CAB-LA prior to the study. Concerns for side effects were the largest anticipated and experienced barrier to CAB-LA. Overall and with subgroups, satisfaction with CAB-LA was high at 24 weeks (97%; 200/206) and 48 weeks (96%; 193/201). Nearly all participants reported that taking CAB-LA was easy at 24 weeks (95%; 195/206) and 48 weeks (99%; 198/201). At CAB-LA discontinuation, 83% (57/69) were likely to extremely likely to recommend CAB-LA to a friend: 80% (20/25) of males, 84% (37/44) of females, 100% (19/19) of youth and 76% (38/50) of older adults.
Conclusions
In rural Uganda and Kenya, over half of participants in the SEARCH trial who were offered choice of oral PrEP/PEP or CAB-LA chose and started CAB-LA during the first 48 weeks. For both males and females and younger and older adults, CAB-LA was both feasible and acceptable to deliver with satisfaction remaining high throughout the study, and nearly all reporting ease of use.
Clinical Trial Number
05549726

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


