Exploring perceptions and preferences for PrEP choice and of an mHealth intervention: insights from the ImPrEP CAB-Brasil study
Abstract
Introduction
Although the efficacy of long-acting injectable cabotegravir (CAB-LA) for pre-exposure prophylaxis (PrEP) is well-known from clinical trials, research is needed to guide effective strategies for its implementation. We describe a qualitative study to assess perceptions and preferences for PrEP choice and acceptability of an mHealth intervention within the ImPrEP CAB Brasil study.
Methods
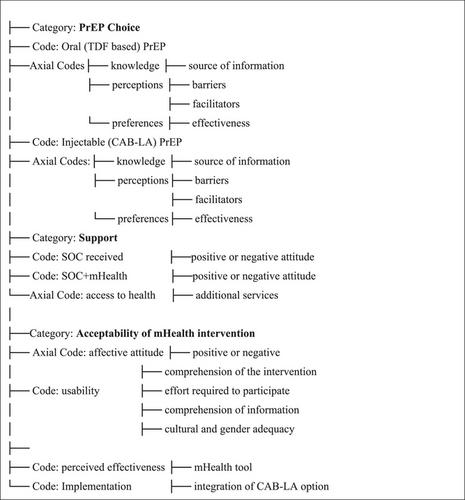
ImPrEP CAB Brasil is an implementation study of same-day delivery of CAB-LA for young sexual and gender minorities (SGM; 18–30 years) in oral PrEP public health clinics in six Brazilian cities. At enrolment, participants received counselling on HIV prevention (SOC) or SOC+mHealth tool to choose between oral or injectable PrEP. The mHealth tool consisted of five videos describing HIV combined prevention including PrEP options. A subset of participants from each site were invited to participate in the qualitative study (October 2023−July 2024). Semi-structured interviews were conducted, recorded and transcribed. Data were fed into ATLAS.ti.24 software. Conventional content analysis was used for coding categories based on an inductive reasoning process.
Results
We conducted 120 interviews (48 SOC and 72 SOC+mHealth; 107 CAB-LA and 13 oral PrEP). Participants reported not knowing about CAB-LA before enrolment; some recently heard from a partner or friend. Reasons for choosing CAB-LA were perceived convenience, practicality, easier adherence to bimonthly injections and higher efficacy compared to oral PrEP. Reasons for not choosing CAB-LA were fear of injections and pain. Reasons for choosing oral PrEP included perspective of less appointments, easiness of daily adherence, access in case of travel and the option to stop immediately if desired or needed. Reasons for not choosing oral PrEP included forgetfulness of daily intake, gastrointestinal side effects, fear of inadvertent exposure and judgement by family. Participants found the mHealth educational tool useful and adequate for PrEP education and decision-making.
Conclusions
Perceptions for PrEP choice among SGM underscore the importance of providing comprehensive information and support towards decision-making processes, so users can have an accurate understanding of each PrEP option, as well as their clinical and social benefits. The mHealth tool was perceived as highly desirable and useful for PrEP education, having the potential to be implemented in HIV prevention services.
Clinical Trial Number
NCT05515770

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


