Structural insights on the differentiation and reversion of conformational changes in SARS-CoV-2 spike protein models across variants occurring from December, 2019 to November, 2021
引用次数: 0
Abstract
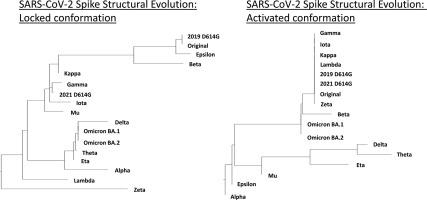
Conformational changes in the SARS-CoV-2 spike protein are critical for understanding viral evolution. In this study, we provide comparative structural and electrostatic analyses across variants, revealing both differentiation and reversion patterns not previously described in locked and activated spike conformations. More specifically, we generated SARS2 spike protein models from the various recorded variants between December, 2019 and November 2021, and performed structural superimposition, dendrogram analyses, and electrostatic mapping. We confirmed which locked and activated conformations differed and reversed between the Original spike protein model and subsequent SARS2 variants and subvariants. Additionally, among the spike protein models of subsequent SARS2 variants and subvariants during December, 2019-November, 2021, we likewise established structural variations and reversions among the locked and activated conformations. Moreover, we established the structural relationship and clustering among the locked and activated conformations of the SARS2 spike protein models. Furthermore, we determined the electrostatic potential of all generated SARS2 spike protein models to establish the surface charge distribution. Taken together, we found that certain locked and activated conformations of the Original SARS2 spike protein models exhibited both structural differences and, surprisingly, reversion when compared to subsequent variants and subvariants. Similarly, structural differentiation and reversion were also observed in the locked and activated conformations across the spike protein models. Additionally, we identified distinct structural clusters within the locked and activated conformations, establishing a structural relationship among certain SARS2 spike protein models. Moreover, we found that during spike evolution reorganization of the surface charge distribution occurs during structural differentiation and reversion.
2019年12月至2021年11月发生变异的SARS-CoV-2刺突蛋白模型构象变化分化和逆转的结构见解
SARS-CoV-2刺突蛋白的构象变化对于理解病毒进化至关重要。在这项研究中,我们提供了跨变体的比较结构和静电分析,揭示了之前未在锁定和激活的尖峰构象中描述的分化和逆转模式。更具体地说,我们从2019年12月至2021年11月期间记录的各种变体中生成了SARS2刺突蛋白模型,并进行了结构叠加、树突图分析和静电作图。我们确认了在原始刺突蛋白模型和随后的SARS2变异体和亚变异体之间锁定和激活的构象不同和逆转。此外,在2019年12月至2021年11月期间的后续SARS2变异体和亚变异体的刺突蛋白模型中,我们同样建立了锁定和激活构象之间的结构变化和逆转。此外,我们建立了SARS2刺突蛋白模型的锁定和激活构象之间的结构关系和聚类。此外,我们测定了所有生成的SARS2刺突蛋白模型的静电电位,以建立表面电荷分布。综上所述,我们发现,与随后的变体和亚变体相比,原始SARS2刺突蛋白模型的某些锁定和激活构象既表现出结构差异,又表现出令人惊讶的逆转。同样,在钉突蛋白模型的锁定和激活构象中也观察到结构分化和逆转。此外,我们在锁定和激活的构象中发现了不同的结构簇,建立了某些SARS2刺突蛋白模型之间的结构关系。此外,我们还发现,在尖刺演化过程中,表面电荷分布的重组发生在结构分化和逆转过程中。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Immunoinformatics (Amsterdam, Netherlands)
Immunology, Computer Science Applications
自引率
0.00%
发文量
0
审稿时长
60 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


