Selective deuteration of terminal olefins with D2O by catalysis of osmium-hydride complexes
IF 4.6
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
An efficient catalytic system for selective deuteration of terminal olefins using D2O as the deuterium source has been developed. A series of osmium hydride complexes of the type OsHX(CO)(PR3)2(L) (where X represents halides) has been evaluated as catalyst precursors for H/D exchange between olefins and D2O. The catalytic activity of the complexes was found to depend on the ligands present. Among the complexes tested, the OsHI(CO)(PPh3)3 complex exhibited excellent catalytic activity for the hydrogen–deuterium (H/D) exchange of terminal olefins with D2O, especially in the presence of acetic acid. The system shows high selectivity for deuteration at terminal double bonds over internal ones. It can catalyze H/D exchange reactions of olefins without causing isomerization and can induce selective H/D exchange at the methine carbon (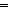 CHR) over the methylidene carbon (
CHR) over the methylidene carbon (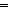 CH2) for substrates RCH
CH2) for substrates RCH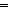 CH2 with a bulky substituent R. Additionally, these reactions can be performed on substrates with various functional groups, making this system useful for selective labeling of olefins in complex molecules.
CH2 with a bulky substituent R. Additionally, these reactions can be performed on substrates with various functional groups, making this system useful for selective labeling of olefins in complex molecules.

氢化锇配合物催化D2O对末端烯烃的选择性氘化反应
开发了一种以D2O为氘源的高效催化体系,用于末端烯烃的选择性氘化。一系列OsHX(CO)(PR3)2(L)型氢化锇配合物(其中X代表卤化物)被评价为烯烃和D2O之间H/D交换的催化剂前驱体。发现配合物的催化活性取决于存在的配体。在所测试的配合物中,OsHI(CO)(PPh3)3配合物对末端烯烃与D2O的氢-氘(H/D)交换表现出优异的催化活性,特别是在乙酸存在的情况下。该体系对末端双键氘化的选择性高于内部双键。它可以催化烯烃的H/D交换反应而不引起异构化,并且可以诱导甲基碳(CHR)与亚甲基碳(CH2)之间的选择性H/D交换,以取代基r较大的RCHCH2为底物。此外,这些反应可以在具有各种官能团的底物上进行,使该体系可用于复杂分子中烯烃的选择性标记。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Chemistry Frontiers
CHEMISTRY, ORGANIC-
CiteScore
7.90
自引率
11.10%
发文量
686
审稿时长
1 months
期刊介绍:
Organic Chemistry Frontiers is an esteemed journal that publishes high-quality research across the field of organic chemistry. It places a significant emphasis on studies that contribute substantially to the field by introducing new or significantly improved protocols and methodologies. The journal covers a wide array of topics which include, but are not limited to, organic synthesis, the development of synthetic methodologies, catalysis, natural products, functional organic materials, supramolecular and macromolecular chemistry, as well as physical and computational organic chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


