Project Management Digitalisation of the Clinical Research at the University Medical Centre: Good Practice of using REDCap as a Digitalisation Tool
IF 4.1
2区 医学
Q2 COMPUTER SCIENCE, INFORMATION SYSTEMS
International Journal of Medical Informatics
Pub Date : 2025-06-28
DOI:10.1016/j.ijmedinf.2025.106028
引用次数: 0
Abstract
Objective
The digital tool REDCap (Research Electronic Data Capture) was implemented at the University Medical Centre Ljubljana (UMCL) with the goal of digitalising and streamlining research processes. This study aimed to assess the efficiency and transparency of clinical research following the implementation of REDCap.
Methods
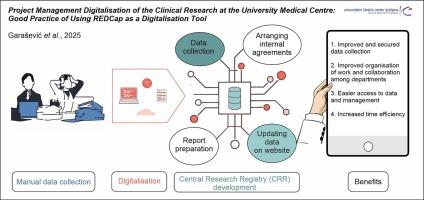
The implementation of REDCap for funded research began in 2021. It comprised four key steps: (I) the initial creation of Central Research Registry, followed by additional functionalities including (II) the establishment of the Central Database for ’Pre-Contract Activities’ for clinical trials; (III) the integration of Reporting on Research Progress directly into the Central Research Registry; and (IV) the development of a semi-automated Workflow for internal agreements.
Results
Between 2021 and 2023, UMCL established a Central Research Registry using REDCap, transitioning from paper-based to digital data collection for over 2,500 research projects. These projects included clinical trials, national and international studies, as well as academic research. In addition to serving as a registry, the central system provided comprehensive data management, streamlined communication, and enhanced collaboration among stakeholders in clinical trial research at UMCL. The implementation of REDCap significantly reduced administrative burden and shortened the time required to finalise clinical trial agreements from 202 to 147 days. It also improved coordination, transparency, and real-time monitoring of research activities, facilitating more efficient research execution. Additionally, the digitalisation of internal agreements processes between researchers and stakeholders within UMCL improved coordination and expedited research execution timelines. Furthermore, REDCap enabled real-time monitoring of research progress, further contributing to the efficiency and transparency of research activities.
Conclusion
The digitalisation of research processes using REDCap improved the organisation and execution of research, leading to greater efficiency and transparency, reduced administrative workload, and enhanced collaboration. This approach contributed to higher-quality research outcomes and ultimately benefited patient care.
大学医学中心临床研究项目管理数字化:使用REDCap作为数字化工具的良好实践
在卢布尔雅那大学医学中心(UMCL)实施了数字工具REDCap(研究电子数据捕获),目的是使研究过程数字化并简化。本研究旨在评估REDCap实施后临床研究的效率和透明度。方法从2021年开始实施REDCap资助研究。它包括四个关键步骤:(I)初步创建中央研究登记处,随后是额外的功能,包括(II)建立临床试验“合同前活动”的中央数据库;(III)将“报告研究进度”直接纳入中央研究注册处;(四)开发内部协议的半自动化工作流。在2021年至2023年期间,UMCL使用REDCap建立了一个中央研究注册中心,为2500多个研究项目从纸质数据收集过渡到数字数据收集。这些项目包括临床试验、国内和国际研究以及学术研究。除了作为注册中心外,中央系统还提供全面的数据管理,简化沟通,并加强UMCL临床试验研究的利益相关者之间的合作。REDCap的实施大大减轻了行政负担,并将完成临床试验协议所需的时间从202天缩短至147天。它还改善了研究活动的协调、透明度和实时监测,促进了更有效的研究执行。此外,UMCL内部研究人员和利益相关者之间的内部协议流程的数字化改善了协调并加快了研究执行时间表。此外,REDCap实现了对研究进展的实时监测,进一步提高了研究活动的效率和透明度。使用REDCap的研究过程数字化改善了研究的组织和执行,提高了效率和透明度,减少了行政工作量,加强了协作。这种方法有助于提高研究结果的质量,并最终使患者护理受益。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

International Journal of Medical Informatics
医学-计算机:信息系统
CiteScore
8.90
自引率
4.10%
发文量
217
审稿时长
42 days
期刊介绍:
International Journal of Medical Informatics provides an international medium for dissemination of original results and interpretative reviews concerning the field of medical informatics. The Journal emphasizes the evaluation of systems in healthcare settings.
The scope of journal covers:
Information systems, including national or international registration systems, hospital information systems, departmental and/or physician''s office systems, document handling systems, electronic medical record systems, standardization, systems integration etc.;
Computer-aided medical decision support systems using heuristic, algorithmic and/or statistical methods as exemplified in decision theory, protocol development, artificial intelligence, etc.
Educational computer based programs pertaining to medical informatics or medicine in general;
Organizational, economic, social, clinical impact, ethical and cost-benefit aspects of IT applications in health care.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


