Cathepsin S mediates the disruption of airway epithelial Pannexin 1 via an Akt-dependent pathway in an LPS-induced murine model of acute lung injury
IF 3.5
3区 生物学
Q3 CELL BIOLOGY
引用次数: 0
Abstract
Airway epithelial dysfunction constitutes a pivotal contributor to the pathogenesis of acute lung injury (ALI). Pannexin 1 (Panx1), a plasma membrane channel activated during epithelial injury, not only helps dampen inflammation but also plays a key role in epithelial repair. Cathepsin S (CTSS) is implicated in ALI pathophysiology. This study investigates the mechanistic link between CTSS and airway epithelial Panx1 dysregulation in ALI pathogenesis. Lipopolysaccharide (LPS) was instilled into the airways of BALB/c mice, followed by intraperitoneal injection of the CTSS inhibitor LY3000328. The effects of recombinant mouse CTSS (rCTSS) were tested in vivo, and Akt inhibition was used to explore the possible mechanism. In vitro, LPS-treated BEAS-2B cells were co-cultured with or without CTSS or Akt inhibitors. LPS exposure significantly increased pulmonary expression of CTSS. Treatment with LY3000328 alleviated the LPS-induced neutrophil accumulation, alveolar permeability and edema. Additionally, we observed decreased expression of Panx1 in the airway epithelium of LPS-exposed mice, accompanied by increased serine phosphorylation of Akt (p-Akt); inhibition of CTSS restored Panx1 and suppressed the p-Akt. Treatment with rCTSS directly downregulated Panx1 expression and induced Akt phosphorylation in the lung, which could be reversed by pharmacological inhibitor of Akt. In BEAS-2B cells, LPS increased CTSS and p-Akt expression alongside decreased levels of junction proteins (E-cadherin and occludin) and Panx1. Blockade of the CTSS/Akt axis restored the disruption of E-cadherin, occludin, and Panx1. Taken together, our data demonstrated that CTSS regulates the dysregulation of airway epithelial Panx1 through the Akt signaling pathway in an LPS-induced ALI model.
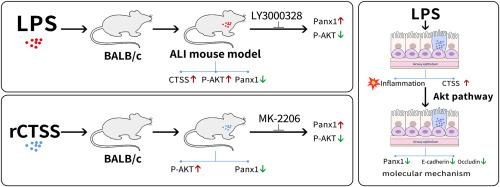
在lps诱导的小鼠急性肺损伤模型中,组织蛋白酶S通过akt依赖途径介导气道上皮Pannexin 1的破坏
气道上皮功能障碍是急性肺损伤(ALI)发病机制的关键因素。Pannexin 1 (Panx1)是上皮损伤时激活的质膜通道,不仅有助于抑制炎症,而且在上皮修复中起关键作用。组织蛋白酶S (CTSS)与ALI的病理生理有关。本研究探讨了CTSS与ALI发病过程中气道上皮Panx1异常之间的机制联系。在BALB/c小鼠气道内灌注脂多糖(LPS),然后腹腔注射CTSS抑制剂LY3000328。在体内测试重组小鼠CTSS (rCTSS)的作用,并通过抑制Akt来探讨其可能的机制。体外,lps处理的BEAS-2B细胞与CTSS或Akt抑制剂共培养或不共培养。LPS暴露显著增加CTSS在肺中的表达。LY3000328可减轻lps诱导的中性粒细胞积聚、肺泡通透性和水肿。此外,我们观察到lps暴露小鼠气道上皮中Panx1的表达降低,同时Akt丝氨酸磷酸化(p-Akt)升高;抑制CTSS恢复Panx1,抑制p-Akt。rCTSS治疗可直接下调Panx1的表达,诱导Akt磷酸化,而Akt的药物抑制剂可逆转这一作用。在BEAS-2B细胞中,LPS增加了CTSS和p-Akt的表达,同时降低了连接蛋白(E-cadherin和occludin)和Panx1的水平。阻断CTSS/Akt轴可恢复E-cadherin、occludin和Panx1的破坏。综上所述,我们的数据表明,在lps诱导的ALI模型中,CTSS通过Akt信号通路调节气道上皮Panx1的失调。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Experimental cell research
医学-细胞生物学
CiteScore
7.20
自引率
0.00%
发文量
295
审稿时长
30 days
期刊介绍:
Our scope includes but is not limited to areas such as: Chromosome biology; Chromatin and epigenetics; DNA repair; Gene regulation; Nuclear import-export; RNA processing; Non-coding RNAs; Organelle biology; The cytoskeleton; Intracellular trafficking; Cell-cell and cell-matrix interactions; Cell motility and migration; Cell proliferation; Cellular differentiation; Signal transduction; Programmed cell death.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


