Percutaneous absorption of climbazole: In vitro data from human skin
IF 3.5
4区 医学
Q1 MEDICINE, LEGAL
引用次数: 0
Abstract
Climbazole is an antifungal substance used as an active ingredient or antimicrobial preservative in pharmaceuticals and personal care products. It is classified by the EU as an acute toxicant (Category 4), and ECHA has recently confirmed its endocrine disruptor concern. Data on climbazole's skin permeability, and consequently its occupational risks, are limited.
The aim of this study was to generate percutaneous absorption data in line with OECD guidelines to support occupational exposure assessment. In vitro experiments using 4.1 μg/cm2 [14C]-labelled climbazole were conducted on freshly excised human skin samples placed in Franz diffusion cells, monitoring absorption over 20 h. The absorption profile data were used to calculate key parameters, including steady-state flux, lag time, and skin permeability coefficient (Kp). The dose distribution across various compartments, including the skin, was evaluated. The individual skin layers were isolated by sequential tape-stripping, followed by epidermis-dermis separation to more precisely measure radioactivity levels. This information was used to predict the potential for further absorption of the dose retained within the skin. The presence of climbazole metabolites in the receptor fluid was also investigated.
The Kp was determined to be 4.7 x 10−3 cm/h. Significant dermal absorption was measured, highlighting potential occupational risks. Climbazole is mainly absorbed with biotransformation: 67 % of the absorbed dose was detected as metabolites. These new percutaneous absorption data will enhance the assessment of the occupational risks associated with dermal exposure to climbazole.
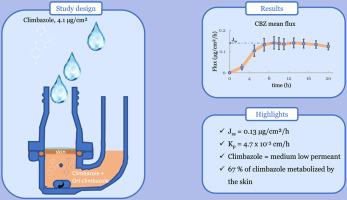
克里巴唑的经皮吸收:来自人体皮肤的体外数据。
克里巴唑是一种抗真菌物质,在药品和个人护理产品中用作活性成分或抗菌防腐剂。它被欧盟列为急性毒物(第4类),ECHA最近确认了其内分泌干扰物的问题。关于克里巴唑的皮肤渗透性及其职业风险的数据是有限的。本研究的目的是生成符合经合组织指导方针的经皮吸收数据,以支持职业暴露评估。用4.1 μg/cm2 [14C]标记的克里巴唑进行体外实验,将新鲜切除的人体皮肤样本置于Franz扩散池中,监测20小时内的吸收情况。吸收剖面数据用于计算关键参数,包括稳态通量、滞后时间和皮肤渗透系数(Kp)。评估了包括皮肤在内的各个隔室的剂量分布。单个皮肤层通过连续的胶带剥离分离,然后是表皮-真皮分离,以更精确地测量放射性水平。这些信息被用来预测残留在皮肤内的剂量进一步吸收的可能性。还研究了受体液中克里巴唑代谢物的存在。Kp为4.7 × 10- 3cm /h。测量了显著的皮肤吸收,突出了潜在的职业风险。克里巴唑主要通过生物转化被吸收,吸收剂量的67%被检测为代谢物。这些新的经皮吸收数据将加强与皮肤接触克里巴唑相关的职业风险评估。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
6.70
自引率
8.80%
发文量
147
审稿时长
58 days
期刊介绍:
Regulatory Toxicology and Pharmacology publishes peer reviewed articles that involve the generation, evaluation, and interpretation of experimental animal and human data that are of direct importance and relevance for regulatory authorities with respect to toxicological and pharmacological regulations in society. All peer-reviewed articles that are published should be devoted to improve the protection of human health and environment. Reviews and discussions are welcomed that address legal and/or regulatory decisions with respect to risk assessment and management of toxicological and pharmacological compounds on a scientific basis. It addresses an international readership of scientists, risk assessors and managers, and other professionals active in the field of human and environmental health.
Types of peer-reviewed articles published:
-Original research articles of relevance for regulatory aspects covering aspects including, but not limited to:
1.Factors influencing human sensitivity
2.Exposure science related to risk assessment
3.Alternative toxicological test methods
4.Frameworks for evaluation and integration of data in regulatory evaluations
5.Harmonization across regulatory agencies
6.Read-across methods and evaluations
-Contemporary Reviews on policy related Research issues
-Letters to the Editor
-Guest Editorials (by Invitation)

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


