Heterogenization of N,N'-bis(salicylidene)-1,2-phenylenediaminocobalt(II) onto functionalized SBA-15 for oxidation of alcohols
IF 2.1
3区 化学
Q3 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
The N,N'-bis(salicylidene)-1,2-phenylenediaminocobalt(II) complex was synthesized by treating bis(salicylidene)phenylenediamine Schiff base with cobaltous chloride and, subsequently, successfully grafting onto amine-functionalized SBA-15 results in a robust heterogeneous catalyst. The structure and composition of the complex were confirmed by the presence of a sharp vibrational band appearing at 402, 518, and 578 cm⁻¹, characteristic of the Co-O bond, along with ¹H NMR and mass spectrometry. The surface grafting of the cobalt complex was evident from FTIR, low-angle powder XRD, TGA, nitrogen adsorption-desorption, and FESEM analyses. The catalytic behavior of homogeneous and heterogenized cobalt complex was evaluated for the oxidation of benzhydrol and various primary and secondary alcohols using t‑butyl hydroperoxide (TBHP) as the oxidant. The heterogeneous system demonstrated excellent activity and reusability, achieving complete conversion of benzhydrol to benzophenone at 90 °C. Studies on different substances showed that the system worked very well for cinnamyl alcohol and had moderate activity with benzyl alcohol, furfuryl alcohol, and cyclohexanol, with the differences due to the unique structures of each substance. The proposed mechanism involves the formation of a cobalt-peroxo intermediate facilitating alcohol oxidation. The catalyst exhibited remarkable stability and retained its activity over multiple cycles, underscoring its potential as an efficient and reusable system for selective alcohol oxidation under mild conditions.
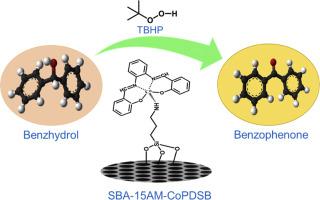
N,N'-双(水杨基)-1,2-苯基二氨基钴(II)在功能化SBA-15上的异质化用于醇的氧化
用氯化钴处理双(水杨基)苯二胺希夫碱,合成了N,N'-双(水杨基)-1,2-苯二氨基钴(II)配合物,并成功接枝到胺功能化的SBA-15上,得到了一种稳定的非均相催化剂。复杂的结构和成分被证实的存在大幅振动乐队出现在402年,518年和578厘米⁻¹,Co-O债券的特征以及¹H核磁共振和质谱。FTIR、低角粉末XRD、TGA、氮气吸附-解吸和FESEM等分析均显示钴配合物的表面接枝。以过氧化氢丁基(TBHP)为氧化剂,考察了均相和异相钴配合物对苯并氢和各种伯、仲醇的氧化行为。该非均相体系表现出良好的活性和可重复使用性,在90°C下实现了苯并氢完全转化为二苯甲酮。对不同物质的研究表明,该体系对肉桂醇的反应效果很好,对苯甲醇、糠醇和环己醇的反应活性中等,但由于每种物质的独特结构而有所不同。提出的机制涉及形成钴-过氧中间体促进醇氧化。该催化剂表现出显著的稳定性,并在多次循环中保持其活性,强调了其在温和条件下作为一种高效和可重复使用的选择性醇氧化系统的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organometallic Chemistry
化学-无机化学与核化学
CiteScore
4.40
自引率
8.70%
发文量
221
审稿时长
36 days
期刊介绍:
The Journal of Organometallic Chemistry targets original papers dealing with theoretical aspects, structural chemistry, synthesis, physical and chemical properties (including reaction mechanisms), and practical applications of organometallic compounds.
Organometallic compounds are defined as compounds that contain metal - carbon bonds. The term metal includes all alkali and alkaline earth metals, all transition metals and the lanthanides and actinides in the Periodic Table. Metalloids including the elements in Group 13 and the heavier members of the Groups 14 - 16 are also included. The term chemistry includes syntheses, characterizations and reaction chemistry of all such compounds. Research reports based on use of organometallic complexes in bioorganometallic chemistry, medicine, material sciences, homogeneous catalysis and energy conversion are also welcome.
The scope of the journal has been enlarged to encompass important research on organometallic complexes in bioorganometallic chemistry and material sciences, and of heavier main group elements in organometallic chemistry. The journal also publishes review articles, short communications and notes.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


