Anion-Mediated Synthesis of Diethynyl-Substituted Doubly N-Confused Dioxohexaphyrin Dyes That Exhibit Enhanced Photoacoustic Signals in the NIR-II Region
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
We herein report the anion-mediated synthesis of diethynyl-substituted doubly N-confused dioxohexaphyrins with hydrogen sulfate anions in an acid condensation of the tripyrrane and silyl-protected propiolaldehyde and subsequent oxidation in a one-pot fashion. Further, functionalization through in situ silyl deprotection and Sonogashira cross-coupling reaction yielded the anisotropically π-extended derivative along a longer molecular axis. The modified hexaphyrin dye exhibited an intense S0 → S1 transition at around 1100 nm in the near-infrared-II region and enhanced photoacoustic signal intensity suitable for high-resolution imaging applications.
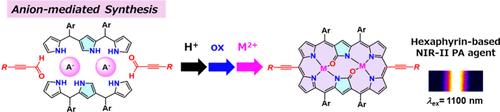
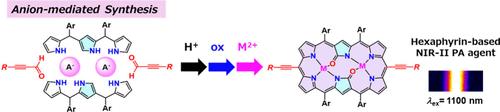
阴离子介导合成在NIR-II区表现出增强光声信号的二乙基取代双n-混淆二氧己葡萄蛋白染料
我们在此报道阴离子介导合成二乙基取代双n-混淆二氧六葡萄素与硫酸氢阴离子在三吡喃和硅基保护丙醛的酸缩合和随后的氧化在一锅方式。此外,通过原位硅基脱保护和Sonogashira交叉偶联反应得到了沿较长分子轴的各向异性π扩展衍生物。改性后的六葡萄蛋白染料在近红外ii区1100 nm处表现出强烈的S0→S1跃迁,光声信号强度增强,适合高分辨率成像应用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


