Identification of an immunomodulatory lncRNA signature associated with immune cell reprogramming in high-grade glioma
IF 5
3区 医学
Q1 BIOTECHNOLOGY & APPLIED MICROBIOLOGY
引用次数: 0
Abstract
High-grade gliomas (HGGs) are among the most aggressive brain tumors in pediatric, adolescent, and young adult (AYA) cancer patients, with a median survival of 12–15 months post-diagnosis. Their poor prognosis is driven by a highly immunosuppressive tumor immune microenvironment (TIME), which inhibits cytotoxic immune infiltration and anti-tumor response. This study investigated the involvement of long non-coding RNAs (lncRNAs) in shaping the immune phenotype of HGGs using two murine models: RCAS-PDGFb representing an immunosuppressive TME, and RCAS-BRAF V600E characterized by a signature more consistent with a pro-inflammatory TME. Transcriptomic analysis of tumor-infiltrating immune cells identified distinct lncRNA signatures associated with immunosuppressive and pro-inflammatory TMEs. Single-cell RNA sequencing and spatial transcriptomics supported context-dependent expression of these lncRNAs in high-grade glioma-associated immune cells, such as myeloid, T, and NK cells, and revealed their spatial distribution within the glioblastoma (GBM) TME. Several lncRNAs were enriched at the tumor edge and within necrotic regions in GBM patient samples, correlating with immunosuppression reprogramming and immune evasion mechanisms. These findings highlight specific immunomodulatory lncRNAs as potential players in the immunosuppressive glioma TME, and likely candidates for future studies aimed at developing novel therapeutic strategies to overcome immune suppression and improve clinical outcomes.
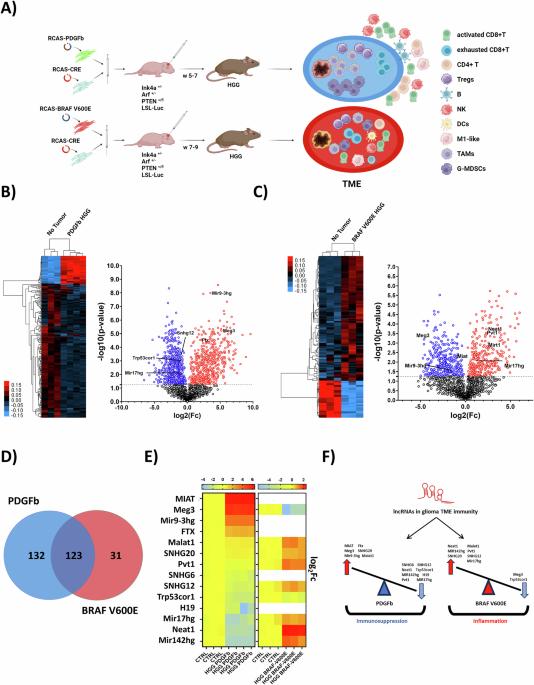
鉴定高级别胶质瘤中与免疫细胞重编程相关的免疫调节lncRNA特征。
高级别胶质瘤(HGGs)是儿童、青少年和年轻人(AYA)癌症患者中最具侵袭性的脑肿瘤之一,诊断后的中位生存期为12-15个月。其不良预后是由高度免疫抑制的肿瘤免疫微环境(TIME)驱动的,它抑制细胞毒性免疫浸润和抗肿瘤反应。本研究利用两种小鼠模型研究了长链非编码rna (lncRNAs)在塑造HGGs免疫表型中的作用:RCAS-PDGFb代表免疫抑制性TME, RCAS-BRAF V600E表征的特征更符合促炎TME。肿瘤浸润免疫细胞的转录组学分析鉴定出与免疫抑制和促炎TMEs相关的不同lncRNA特征。单细胞RNA测序和空间转录组学支持这些lncrna在高级别胶质瘤相关免疫细胞(如髓细胞、T细胞和NK细胞)中的上下文依赖性表达,并揭示了它们在胶质母细胞瘤(GBM) TME中的空间分布。在GBM患者样本中,肿瘤边缘和坏死区域富集了几种lncrna,与免疫抑制重编程和免疫逃避机制相关。这些发现强调了特异性免疫调节lncrna在免疫抑制性胶质瘤TME中的潜在作用,以及未来研究的可能候选物,旨在开发新的治疗策略来克服免疫抑制并改善临床结果。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cancer gene therapy
医学-生物工程与应用微生物
CiteScore
10.20
自引率
0.00%
发文量
150
审稿时长
4-8 weeks
期刊介绍:
Cancer Gene Therapy is the essential gene and cellular therapy resource for cancer researchers and clinicians, keeping readers up to date with the latest developments in gene and cellular therapies for cancer. The journal publishes original laboratory and clinical research papers, case reports and review articles. Publication topics include RNAi approaches, drug resistance, hematopoietic progenitor cell gene transfer, cancer stem cells, cellular therapies, homologous recombination, ribozyme technology, antisense technology, tumor immunotherapy and tumor suppressors, translational research, cancer therapy, gene delivery systems (viral and non-viral), anti-gene therapy (antisense, siRNA & ribozymes), apoptosis; mechanisms and therapies, vaccine development, immunology and immunotherapy, DNA synthesis and repair.
Cancer Gene Therapy publishes the results of laboratory investigations, preclinical studies, and clinical trials in the field of gene transfer/gene therapy and cellular therapies as applied to cancer research. Types of articles published include original research articles; case reports; brief communications; review articles in the main fields of drug resistance/sensitivity, gene therapy, cellular therapy, tumor suppressor and anti-oncogene therapy, cytokine/tumor immunotherapy, etc.; industry perspectives; and letters to the editor.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


