Dysfunctional pericellular hyaluronan deposition contributes to attenuated CD44/EGFR co-localization and impaired myofibroblast differentiation in chronic wound fibroblasts
IF 3.5
3区 生物学
Q3 CELL BIOLOGY
引用次数: 0
Abstract
Non-healing chronic wounds, such as venous ulcers and pressure sores, represent significant causes of patient morbidity and financial burden to Healthcare Services worldwide. During normal healing, dermal fibroblasts (DFs) mediate numerous responses to promote wound closure. However, phenotypic changes induced within chronic wound environments lead to dysfunctional fibroblast functions, which facilitate non-healing. Although the processes underlying impaired proliferative and migratory responses in chronic wound fibroblasts (CWFs) are established, the mechanisms that mediate impaired CWF-myofibroblast differentiation remain poorly understood. Fibroblast-myofibroblast differentiation is induced by transforming growth factor-β1 (TGF-β1) and downstream classical Smad2/3 and non-classical epidermal growth factor receptor (EGFR)/ERK1/2 signaling, initiated through hyaluronan (HA) receptor (CD44) binding to EGFR and dependent on elevated HA synthesis and its pericellular accumulation. Here, we demonstrate that these signaling pathways are dysregulated in venous ulcer- and pressure sore-derived CWFs, compared to DFs. CWFs exhibit increased susceptibilities to cellular senescence and impaired myofibroblast differentiation, accompanied by defective lysosomal/endosomal activities and dysfunctional activation of the HA/CD44/EGFR pathway. Irrespective of wound source, CWFs exhibited increased HAS1 versus HAS2 expression, altered HAS1 and HAS2 intracellular localization, and deregulated hyaladherin (CD44, TSG-6, and IαI heavy chain motifs, HC3, HC4 and HC5) induction, following TGF-β1 stimulation. These events attenuated HA pericellular coat formation and CD44/EGFR co-localization within membrane lipid rafts, essential for myofibroblast development. Our findings suggest that aberrant HAS1 and HAS2 expression and distributions cause reduced pericellular hyaluronan deposition, leading to attenuated CD44/EGFR co-localization and dysfunctional CWF-myofibroblast differentiation, which contributes to the impaired closure and healing of chronic wounds.
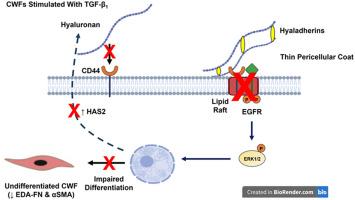
细胞周围透明质酸沉积功能障碍导致慢性伤口成纤维细胞CD44/EGFR共定位减弱和肌成纤维细胞分化受损。
无法愈合的慢性伤口,如静脉溃疡和压疮,是造成患者发病率和全球卫生保健服务负担的重要原因。在正常愈合过程中,真皮成纤维细胞(DFs)介导许多促进伤口愈合的反应。然而,在慢性伤口环境中诱导的表型变化导致成纤维细胞功能失调,从而促进不愈合。虽然慢性伤口成纤维细胞(cwf)增殖和迁移反应受损的过程已经确定,但介导cwf -肌成纤维细胞分化受损的机制仍然知之甚少。成纤维细胞-肌成纤维细胞分化是由转化生长因子-β1 (TGF-β1)和下游经典Smad2/3和非经典表皮生长因子受体(EGFR)/ERK1/2信号诱导的,通过透明质酸(HA)受体(CD44)与EGFR结合启动,依赖于HA合成的升高及其在细胞周围的积累。在这里,我们证明,与DFs相比,这些信号通路在静脉溃疡和压疮衍生的CWFs中失调。CWFs表现出对细胞衰老和肌成纤维细胞分化的易感性增加,并伴有溶酶体/内体活性缺陷和HA/CD44/EGFR通路激活功能障碍。无论伤口来源如何,在TGF-β1刺激后,cwf均表现出HAS1与HAS2表达增加,HAS1和HAS2细胞内定位改变,透明质粘附素(CD44、TSG-6和i - α i重链基序、HC3、HC4和HC5)诱导失调。这些事件减弱了HA细胞外膜的形成和CD44/EGFR在膜脂筏内的共定位,这对肌成纤维细胞的发育至关重要。我们的研究结果表明,异常的HAS1和HAS2表达和分布导致细胞周围透明质酸沉积减少,导致CD44/EGFR共定位减弱和cwf -肌成纤维细胞分化功能障碍,这有助于慢性伤口的闭合和愈合受损。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Experimental cell research
医学-细胞生物学
CiteScore
7.20
自引率
0.00%
发文量
295
审稿时长
30 days
期刊介绍:
Our scope includes but is not limited to areas such as: Chromosome biology; Chromatin and epigenetics; DNA repair; Gene regulation; Nuclear import-export; RNA processing; Non-coding RNAs; Organelle biology; The cytoskeleton; Intracellular trafficking; Cell-cell and cell-matrix interactions; Cell motility and migration; Cell proliferation; Cellular differentiation; Signal transduction; Programmed cell death.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


