Mental well-being in prostate cancer: A multi-institutional prospective cohort study
Abstract
Objective
To investigate the patient, treatment and oncological prognostic factors for multiple mental well-being outcomes in prostate cancer.
Patient and Methods
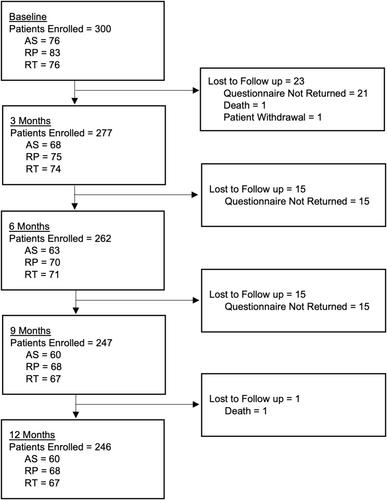
The MIND-P study was a multi-institutional prospective cohort study recruiting newly diagnosed prostate cancer patients for 12 months post-diagnosis across eight centres. Periodic data collection evaluated mental, physical and social well-being measures incorporating five mental well-being outcomes selected based on prior research as important measures in patients with prostate cancer. This included depression, anxiety, fear of recurrence, body image, and masculinity. Treatment, patient, and oncological prognostic factors for developing significant well-being symptoms were evaluated along with symptom trajectories.
Results
Of 300 patients recruited, 13.7% and 11.0% developed depression or anxiety symptoms, with 45.0% developing at least one significant mental well-being symptom. Those undergoing hormone monotherapy had higher depression scores from 6 months post-diagnosis (all p < 0.05), with prostatectomy patients having poorer body image and masculinity scores, when compared with surveillance patients (all p < 0.02). Metastatic disease at diagnosis was associated with increased depression, anxiety and fear of cancer recurrence. Patient factors for poorer mental well-being included younger age, a previous psychiatric history, social deprivation, poorer baseline mental health symptoms and poorer baseline sexual and urinary function. Symptom trajectory analysis demonstrated the increasing symptom load in body image and masculine self-esteem experienced post any active treatment modality, with more stable scores for other mental well-being measures.
Conclusion
A high incidence of multiple mental well-being issues was identified post-diagnosis, highlighting their individual importance during follow-up. Baseline mental and functional symptoms, a previous psychiatric history and stage at diagnosis appear to be particularly important prognostic factors for the development of significant symptoms. A comprehensive initial biopsychosocial assessment incorporating these could identify high-risk patients for improved monitoring and subsequent support.
ClinicalTrials.gov number - NCT04647474.



 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


