NMR insights into the behavior of bis-His motifs toward copper ions: A study using mono-N-methylated histidines
IF 3.2
2区 化学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
The bis-histidine (bis-His) motif, formed by two adjacent histidines, plays a central role in metal coordination within biological systems. Its versatility stems from the imidazole ring of histidine, which offers two distinct nitrogen donors (Nδ/Nπ and Nε/Nτ). This motif contributes to enzymatic activity, redox processes, and metal homeostasis in proteins and peptides. To investigate its coordination properties, we developed a four-point comparative model based on the Aβ12–16 pentapeptide, introducing selective mono-N-methylation of histidine residues. This strategy enabled a detailed NMR-based characterization of the bis-His motif in the presence of Cu (II) and Cu (I). For Cu(II), distinct paramagnetic relaxation effects were observed depending on the methylation site, reflecting differences in metal–ligand coordination sphere. Similarly, variations in chemical shift induced by Ag(I), used as a probe for Cu(I), confirmed differential interactions with the imidazole nitrogens. The His-His pair was found to act as a structurally flexible binding site able to accommodate both copper oxidation state. Our findings highlight a clear preference for Nπ coordination, with specific patterns of interaction depending on the oxidation state and histidine modification. Reactivity studies in the presence of the endogenous antioxidant glutathione (GSH) further confirmed the role of histidine coordination within the bis-His motif in Cu(II)/Cu(I) redox cycling. Notably, the redox behavior was influenced by the specific imidazole nitrogen involved in metal coordination, with distinct reactivity patterns observed depending on whether the Nπ or Nτ nitrogen was engaged.
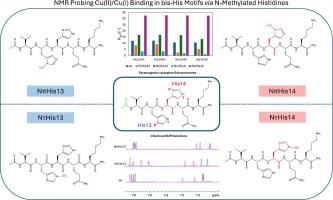
核磁共振洞察双- his基序对铜离子的行为:使用单n -甲基化组氨酸的研究
双组氨酸(bis-His)基序由两个相邻的组氨酸组成,在生物系统中的金属配位中起着核心作用。它的多功能性源于组氨酸的咪唑环,它提供两种不同的氮供体(Nδ/Nπ和Nε/Nτ)。这个基序有助于酶活性、氧化还原过程和蛋白质和肽中的金属稳态。为了研究其配位特性,我们建立了一个基于a β12 - 16五肽的四点比较模型,引入了组氨酸残基的选择性单n甲基化。该策略能够在Cu (II)和Cu (I)存在下对双his基序进行详细的基于核磁共振的表征。对于Cu(II),根据甲基化位点的不同,观察到不同的顺磁弛豫效应,反映了金属配体配位球的差异。同样,由Ag(I)引起的化学位移变化,作为Cu(I)的探针,证实了与咪唑氮的差异相互作用。发现His-His对作为一种结构灵活的结合位点,能够适应铜的氧化态。我们的研究结果强调了对Nπ配位的明显偏好,其特定的相互作用模式取决于氧化态和组氨酸修饰。内源性抗氧化剂谷胱甘肽(GSH)存在下的反应性研究进一步证实了双his基序中组氨酸配位在Cu(II)/Cu(I)氧化还原循环中的作用。值得注意的是,氧化还原行为受到参与金属配位的特定咪唑氮的影响,根据是否参与Nπ或Nτ氮,观察到不同的反应模式。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Inorganic Biochemistry
生物-生化与分子生物学
CiteScore
7.00
自引率
10.30%
发文量
336
审稿时长
41 days
期刊介绍:
The Journal of Inorganic Biochemistry is an established international forum for research in all aspects of Biological Inorganic Chemistry. Original papers of a high scientific level are published in the form of Articles (full length papers), Short Communications, Focused Reviews and Bioinorganic Methods. Topics include: the chemistry, structure and function of metalloenzymes; the interaction of inorganic ions and molecules with proteins and nucleic acids; the synthesis and properties of coordination complexes of biological interest including both structural and functional model systems; the function of metal- containing systems in the regulation of gene expression; the role of metals in medicine; the application of spectroscopic methods to determine the structure of metallobiomolecules; the preparation and characterization of metal-based biomaterials; and related systems. The emphasis of the Journal is on the structure and mechanism of action of metallobiomolecules.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


