Stereoselective Synthesis of Delgocitinib and Its Diastereomer Using Organocatalyzed Michael Addition
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
Herein, we describe the stereoselective synthesis of delgocitinib and its diastereomer. Delgocitinib, a Janus kinase (JAK) inhibitor, is characterized by a diaza-spirocyclic structure consisting of pyrrolidine and azetidine rings. The organocatalyzed Michael addition of propanal with fumarate or maleimide facilitated the formation of contiguous tertiary stereogenic centers, leading to the stereoselective production of each diastereomer. Intramolecular C–H amination reactions introduced a nitrogen atom on one of the tertiary carbons, and subsequent azetidine formation constructed the spirocyclic system.

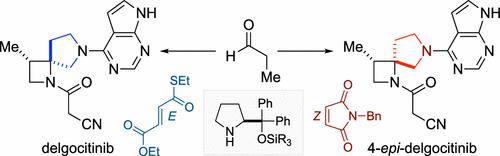
有机催化Michael加成法立体选择性合成德尔古西替尼及其非对映体
本文描述了德尔古西替尼及其非对映体的立体选择性合成。Delgocitinib是一种Janus激酶(JAK)抑制剂,具有由吡咯烷和氮杂啶环组成的重氮-螺旋环结构。丙醛与富马酸酯或马来酰亚胺的有机催化迈克尔加成促进了连续三级立体中心的形成,导致每种非对映体的立体选择性生产。分子内C-H胺化反应在其中一个叔碳上引入了一个氮原子,随后氮杂啶的形成构建了螺环体系。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


