Early Endosome Disturbance and Endolysosomal Pathway Dysfunction in Duchenne Muscular Dystrophy
IF 3.6
2区 医学
Q1 PATHOLOGY
引用次数: 0
Abstract
Duchenne muscular dystrophy (DMD) is a lethal dystrophy characterized by the progressive loss of muscle fibers caused by mutations in DMD gene and absence of the dystrophin protein. Although autophagy and lysosome biogenesis defects have been described in DMD muscles, the endosomal pathway has never been studied. The current study revealed an association of impaired lysosome formation with altered acidification and reduced degradative function of the endolysosomal pathway in muscle cells derived from patients with DMD. Early endosomes were increased in these cells as well as in muscle biopsies from patients with DMD and two animal models of DMD, mdx mice and golden retriever muscular dystrophy dogs. These abnormalities occurred due to the lack of dystrophin per se and could be correlated with disease progression and severity. An abnormal up-regulation of the Rab5 GTPase protein, one key actor of early endosomal biogenesis and fusion, was identified in the three DMD models, which may underlie the endosomal defects. Finally, Rab5 knockdown in human DMD muscle cells as well as dystrophin restoration in golden retriever muscular dystrophy dogs normalized Rab5 expression levels and rescued endosomal abnormalities. This study unveiled a defect in a pathway essential for muscle homeostasis and for the efficacy of DMD therapies.
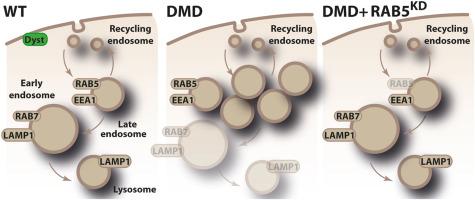
杜氏肌营养不良的早期内溶酶体紊乱和内溶酶体通路功能障碍。
杜氏肌营养不良症(DMD)是一种致命的营养不良症,其特征是由DMD基因突变和肌营养不良蛋白缺失引起的肌肉纤维进行性损失。虽然自噬和溶酶体生物发生缺陷已经在DMD肌肉中被描述,但内体途径从未被研究过。在这里,我们发现,来自DMD患者的肌肉细胞中,溶酶体形成受损与内溶酶体途径的酸化改变和降解功能降低有关。我们的数据表明,在这些细胞中,以及在DMD患者和两种DMD动物模型mdx小鼠和GRMD狗的肌肉活检中,早期内体增加。我们确定这些异常是由于缺乏肌营养不良蛋白本身,并可能与疾病进展和严重程度相关。我们进一步发现,在三种DMD模型中,Rab5 GTPase蛋白异常上调,这是早期内体生物发生和融合的一个关键因素,可能是内体缺陷的基础。最后,我们证明了Rab5在人DMD肌肉细胞中的敲除以及GRMD犬的肌营养不良蛋白恢复,使Rab5表达水平正常化并挽救内体异常。这项研究揭示了肌肉稳态和DMD治疗效果所必需的途径中的缺陷。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.40
自引率
0.00%
发文量
178
审稿时长
30 days
期刊介绍:
The American Journal of Pathology, official journal of the American Society for Investigative Pathology, published by Elsevier, Inc., seeks high-quality original research reports, reviews, and commentaries related to the molecular and cellular basis of disease. The editors will consider basic, translational, and clinical investigations that directly address mechanisms of pathogenesis or provide a foundation for future mechanistic inquiries. Examples of such foundational investigations include data mining, identification of biomarkers, molecular pathology, and discovery research. Foundational studies that incorporate deep learning and artificial intelligence are also welcome. High priority is given to studies of human disease and relevant experimental models using molecular, cellular, and organismal approaches.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


