A food-sensitive olfactory circuit drives anticipatory satiety
IF 20.8
1区 医学
Q1 ENDOCRINOLOGY & METABOLISM
引用次数: 0
Abstract
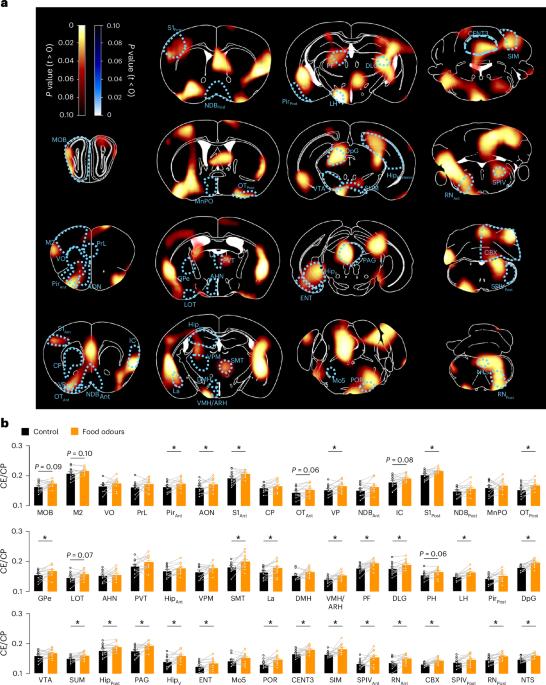
Food sensory perception has emerged as a potent regulator of specialized feeding circuits; yet, the consequences on feeding behaviour and the underlying neuronal basis remain poorly understood. Here, we reveal a sensory pathway that co-ordinately integrates food odours to control forthcoming nutrient intake in male mice. Unbiased whole-brain mapping of food odour-induced brain activity revealed a potent activation of the medial septum (MS), where food odours selectively activate MS glutamatergic neurons (MSVGLUT2). Activity dynamics of MSVGLUT2 neurons uncovered a biphasic modulation of their neuronal activity with a transient activation after detection of food odours and a long-lasting inhibition following food ingestion, independent of the caloric value and identity of the food. MSVGLUT2 neurons receive direct projections from the olfactory bulb (OB) and acute optogenetic stimulation of OB→MS projections selectively before food ingestion decreased feeding in lean mice. However, acute OB→MS optogenetic stimulation in diet-induced obese mice failed to reduce feeding, suggesting the involvement of this pathway in calorie-rich diet-induced hyperphagia and obesity development. Altogether, our study uncovered a sensory circuit by which the organism integrates olfactory food cues to prime satiety at the outset of a meal. The authors describe a sensory circuit involving the medial septum (MS), where MS glutamatergic neurons integrate food odours to prime satiety and regulate nutrient intake.

对食物敏感的嗅觉回路驱动预期饱腹感
食物感官知觉已经成为专门喂养回路的有力调节器;然而,对进食行为的影响和潜在的神经元基础仍然知之甚少。在这里,我们揭示了一个感官途径,协调整合食物气味来控制即将到来的营养摄入在雄性小鼠。无偏倚的全脑食物气味诱导的大脑活动图显示了内侧隔(MS)的有效激活,其中食物气味选择性地激活MS谷氨酸能神经元(MSVGLUT2)。MSVGLUT2神经元的活动动力学揭示了其神经元活动的双相调节,即在检测到食物气味后的短暂激活和食物摄入后的长期抑制,与食物的热值和特性无关。瘦小鼠MSVGLUT2神经元接受嗅球(OB)的直接投射和选择性OB→MS投射的急性光遗传刺激。然而,在饮食诱导的肥胖小鼠中,急性OB→MS光遗传刺激未能减少摄食,这表明该途径参与了高热量饮食诱导的暴饮暴食和肥胖的发展。总之,我们的研究揭示了一个感官回路,通过这个回路,生物体将嗅觉食物线索整合到用餐开始时的初始饱腹感中。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature metabolism
ENDOCRINOLOGY & METABOLISM-
CiteScore
27.50
自引率
2.40%
发文量
170
期刊介绍:
Nature Metabolism is a peer-reviewed scientific journal that covers a broad range of topics in metabolism research. It aims to advance the understanding of metabolic and homeostatic processes at a cellular and physiological level. The journal publishes research from various fields, including fundamental cell biology, basic biomedical and translational research, and integrative physiology. It focuses on how cellular metabolism affects cellular function, the physiology and homeostasis of organs and tissues, and the regulation of organismal energy homeostasis. It also investigates the molecular pathophysiology of metabolic diseases such as diabetes and obesity, as well as their treatment. Nature Metabolism follows the standards of other Nature-branded journals, with a dedicated team of professional editors, rigorous peer-review process, high standards of copy-editing and production, swift publication, and editorial independence. The journal has a high impact factor, has a certain influence in the international area, and is deeply concerned and cited by the majority of scholars.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


