Raman spectroscopic signatures of amyloid fibrils: Insights into structural and biochemical changes in human tissues
IF 2.2
3区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Amyloid fibrils, characterized by β-sheet-rich protein aggregates, are closely associated with various diseases. Understanding the structural and biochemical changes in amyloid formation requires detailed characterization of their Raman spectroscopic signatures. This study evaluated the application of Raman spectroscopy, utilizing a 532-nm laser excitation source, for differentiating amyloid from normal tissues. Raman spectroscopy effectively identifies protein secondary structures and distinguishes normal tissues from amyloid-containing tissues, offering potential for real-time diagnosis. A total of 13 amyloid tissue samples (heart, kidney, and thyroid) and 9 normal controls were analyzed. Key spectral differences were observed in the amide I (∼1660 cm−1) and amide III (∼1300 cm−1) regions, characteristic of β-sheet structures in amyloid fibrils. Spatially resolved Raman spectra revealed molecular heterogeneity between amide and lipid components in amyloid deposits. Ratiometric analysis further supported this, demonstrating significant differences in the amide-to-lipid ratio (with attributed significant peak intensities at 1660 cm−1 for amide I and 1440 cm−1 for lipids) between amyloid and control tissues. Statistical analysis (Mann-Whitney U test, p = 0.006) confirmed significant differences in amide group intensities between amyloid and control tissues. These findings highlight Raman spectroscopy as a promising tool for real-time identification and characterization of amyloid deposits, with potential clinical applications in diagnosing amyloid-related diseases.
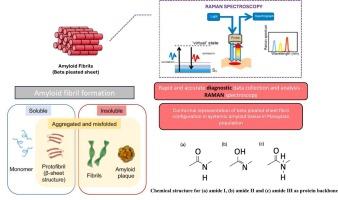
淀粉样蛋白原纤维的拉曼光谱特征:对人体组织结构和生化变化的见解
淀粉样原纤维与多种疾病密切相关,其特征是富含β-薄片的蛋白聚集体。了解淀粉样蛋白形成的结构和生化变化需要详细表征其拉曼光谱特征。本研究评估了利用532 nm激光激发源的拉曼光谱在区分淀粉样蛋白和正常组织中的应用。拉曼光谱可以有效地识别蛋白质二级结构,并将正常组织与含有淀粉样蛋白的组织区分开来,为实时诊断提供了可能。分析了13例(心脏、肾脏和甲状腺)淀粉样蛋白组织样本和9例正常对照。在酰胺I (~ 1660 cm−1)和酰胺III (~ 1300 cm−1)区域观察到关键的光谱差异,这是淀粉样蛋白原纤维中β片结构的特征。空间分辨拉曼光谱揭示了淀粉样蛋白沉积物中酰胺和脂质组分之间的分子异质性。比率分析进一步支持了这一点,证明淀粉样蛋白和对照组织之间酰胺与脂质比率(酰胺I的峰值强度为1660 cm−1,脂质峰值强度为1440 cm−1)存在显著差异。统计分析(Mann-Whitney U检验,p = 0.006)证实淀粉样蛋白和对照组织之间酰胺组强度存在显著差异。这些发现突出了拉曼光谱作为实时识别和表征淀粉样蛋白沉积的有前途的工具,在诊断淀粉样蛋白相关疾病方面具有潜在的临床应用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Biophysical chemistry
生物-生化与分子生物学
CiteScore
6.10
自引率
10.50%
发文量
121
审稿时长
20 days
期刊介绍:
Biophysical Chemistry publishes original work and reviews in the areas of chemistry and physics directly impacting biological phenomena. Quantitative analysis of the properties of biological macromolecules, biologically active molecules, macromolecular assemblies and cell components in terms of kinetics, thermodynamics, spatio-temporal organization, NMR and X-ray structural biology, as well as single-molecule detection represent a major focus of the journal. Theoretical and computational treatments of biomacromolecular systems, macromolecular interactions, regulatory control and systems biology are also of interest to the journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


