ACSL4 knockdown inhibits colorectal cancer progression through stimulating anti-tumor immunity
IF 7.7
2区 医学
Q1 Biochemistry, Genetics and Molecular Biology
引用次数: 0
Abstract
Background: Long-chain acyl-CoA synthetase 4 (ACSL4), a crucial modulator of ferroptosis, is associated with tumor progression, though its impact on colorectal cancer (CRC) immune dynamics is not fully understood.
Methods: ACSL4 expression was analyzed in CRC tissues and correlated with patient prognosis. Effects of ACSL4 were evaluated in CRC cells in vitro and in subcutaneous and orthotopic CRC models. Flow cytometry and immunofluorescence were used to evaluate immune cell infiltration. RNA sequencing and RT-PCR were employed to identify ACSL4-regulated signaling pathways. The effect of ACSL4 silencing on PD-L1 blockade efficacy was also examined.
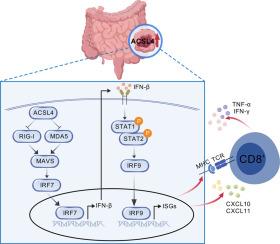
Results: ACSL4 levels were markedly increased in CRC and linked to unfavorable patient outcomes. While ACSL4 knockdown had no direct effect on CRC cell proliferation, it significantly suppressed tumor growth in immunocompetent mice. ACSL4 depletion enhanced CD3⁺ and CD8⁺ T cell infiltration and upregulated chemokines (CXCL10, CXCL11) and antigen presentation genes (H2k1, TAP1, TAPBP). Transcriptomic analysis highlighted activation of the RIG-I-MAVS-driven type I interferon pathway. Co-culture assays demonstrated that ACSL4 knockdown promoted CD8⁺ T cell activation, and ACSL4-deficient tumors exhibited enhanced responsiveness to PD-L1 blockade.
Conclusions: ACSL4 suppresses anti-tumor immunity in CRC by modulating the RIG-I-MAVS-IFN pathway, highlighting ACSL4 as a promising target for CRC immunotherapy.
ACSL4敲低通过刺激抗肿瘤免疫抑制结直肠癌进展
背景:长链酰基辅酶a合成酶4 (ACSL4)是铁凋亡的重要调节因子,与肿瘤进展有关,尽管其对结直肠癌(CRC)免疫动力学的影响尚不完全清楚。方法:分析ACSL4在结直肠癌组织中的表达及其与患者预后的关系。在体外、皮下和原位CRC模型中评估ACSL4对CRC细胞的影响。流式细胞术和免疫荧光法检测免疫细胞浸润情况。采用RNA测序和RT-PCR技术鉴定acsl4调控的信号通路。我们还研究了ACSL4沉默对PD-L1阻断效果的影响。结果:ACSL4水平在结直肠癌中显著升高,并与患者的不良预后相关。虽然ACSL4敲除对CRC细胞增殖无直接影响,但在免疫功能正常的小鼠中可显著抑制肿瘤生长。ACSL4缺失增强CD3 +和CD8 + T细胞浸润,上调趋化因子(CXCL10、CXCL11)和抗原呈递基因(H2k1、TAP1、TAPBP)。转录组学分析强调了rig -I- mavs驱动的I型干扰素途径的激活。共培养实验表明,ACSL4敲低促进了CD8 + T细胞的活化,ACSL4缺陷肿瘤对PD-L1阻断的反应性增强。结论:ACSL4通过调节RIG-I-MAVS-IFN通路抑制结直肠癌的抗肿瘤免疫,表明ACSL4是结直肠癌免疫治疗的一个有希望的靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Neoplasia
医学-肿瘤学
CiteScore
9.20
自引率
2.10%
发文量
82
审稿时长
26 days
期刊介绍:
Neoplasia publishes the results of novel investigations in all areas of oncology research. The title Neoplasia was chosen to convey the journal’s breadth, which encompasses the traditional disciplines of cancer research as well as emerging fields and interdisciplinary investigations. Neoplasia is interested in studies describing new molecular and genetic findings relating to the neoplastic phenotype and in laboratory and clinical studies demonstrating creative applications of advances in the basic sciences to risk assessment, prognostic indications, detection, diagnosis, and treatment. In addition to regular Research Reports, Neoplasia also publishes Reviews and Meeting Reports. Neoplasia is committed to ensuring a thorough, fair, and rapid review and publication schedule to further its mission of serving both the scientific and clinical communities by disseminating important data and ideas in cancer research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


