Synthesis and structure of (E)-3,4,5-trihydroxy-N′-(3,4,5-trimethoxybenzylidene)benzohydrazide monohydrate
IF 0.6
Q4 CRYSTALLOGRAPHY
Acta Crystallographica Section E: Crystallographic Communications
Pub Date : 2025-06-01
DOI:10.1107/S2056989025004001
引用次数: 0
Abstract
In the structure of the title compound, C17H18N2O7·H2O, relatively strong bifurcated and simple hydrogen-bond interactions are present, together with extended van der Waals interactions. A hydrogen-bond coordination analysis suggests that polymorphs of the title structure may exist.
In the structure of the title compound, C17H18N2O7·H2O, hydrogen bonds link three molecules to the water molecule. Additional hydrogen-bonding interactions connect two molecules via the amide nitrogen donor and two methoxy oxygen acceptors. This three-dimensional hydrogen-bond network has no particular directionality, and a slight disorder of two methoxy groups is observed. According to force-field calculations, weaker but more extended van der Waals interactions show a larger stabilization effect than the hydrogen-bond interactions, while hydrogen-bond analysis suggests that polymorphs of the compound may be found in the future.
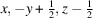
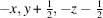
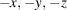
(E)-3,4,5-三羟基- n '-(3,4,5-三甲基-氧基苯并肼)一水合物的合成与结构
在标题化合物C17H18N2O7·H2O的结构中,氢键将三个分子连接到水分子上。额外的氢键相互作用通过酰胺氮供体和两个甲基氧受体连接两个分子。这种三维氢键网络没有特定的方向性,并且观察到两个甲氧基的轻微紊乱。根据力场计算,较弱但扩展更广的范德华相互作用比氢键相互作用表现出更大的稳定效应,而氢键分析表明,化合物的多晶态可能在未来被发现。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Acta Crystallographica Section E: Crystallographic Communications
Chemistry-Chemistry (all)
CiteScore
1.90
自引率
0.00%
发文量
351
审稿时长
3 weeks
期刊介绍:
Acta Crystallographica Section E: Crystallographic Communications is the IUCr''s open-access structural communications journal. It provides a fast, simple and easily accessible publication mechanism for crystal structure determinations of inorganic, metal-organic and organic compounds. The electronic submission, validation, refereeing and publication facilities of the journal ensure rapid and high-quality publication of fully validated structures. The primary article category is Research Communications; these are peer-reviewed articles describing one or more structure determinations with appropriate discussion of the science.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


