Synthesis and structural characterization of the dichloride complex formed by carboxy-functionalized Cu(diazacyclam)2+ cation and its heterometallic coordination polymer with CdCl2
IF 0.6
Q4 CRYSTALLOGRAPHY
Acta Crystallographica Section E: Crystallographic Communications
Pub Date : 2025-06-01
DOI:10.1107/S2056989025003792
引用次数: 0
Abstract
The coordination polyhedra of the CuII ions in molecular complex I and coordination polymer II represent tetragonally elongated trans-CuN4Cl2 and trans-CuN4(H2O)Cl octahedra, respectively, with the four N atoms of the macrocyclic ligand forming the equatorial plane and monodentate ligands occupying the axial positions. The coordination polyhedron of the CdII ion in II is a CdO4Cl2 distorted octahedron formed by two bidentately coordinated deprotonated carboxylic groups of different CuII macrocyclic cations and two chloride anions, one of which displays a bridging function.
The asymmetric unit of the complex [3,10-bis(3-carboxypropyl)-1,3,5,8,10,12-hexaazacyclotetradecane-κ4N1,N5,N8,N12]dichloridocopper(II), [CuCl2(C16H34N6O4)] or [Cu(H2L)Cl2] (I), consists of a centrosymmetric macrocyclic CuII dication and a chloride anion. The components of the heterometallic compound poly[[aqua[μ3-3,10-bis(3-carboxypropyl)-1,3,5,8,10,12-hexaazacyclotetradecane-κ4N1,N5,N8,N12:κ2O,O′:κ2O′′,O′′′]-μ-chlorido-copper(II)cadmium(II] 1.25-hydrate], {[CuCd(C16H32N6O4)Cl2(H2O)]·1.25H2O}n or {[CuCd(L)(H2O)Cl2]·1.25H2O}n (II) are [Cu(L)(H2O)] moieties coordinated to CdCl2 units via the deprotonated carboxylic groups of the macrocycle, and four water molecules of crystallization with partial occupancies. In each compound, the CuII ion coordinates in the equatorial plane by the four secondary N atoms of the macrocyclic ligand, which adopts the most energetically stable trans-III conformation, and two mutually trans axial ligands in tetragonally elongated trans-CuN4Cl2 and trans-CuN4(H2O)Cl octahedral geometries in I and II, respectively. The coordination environment of the CdII ion in II is a CdO4Cl2 distorted octahedron formed by two bidentately coordinated deprotonated carboxylic groups of different macrocycles and two chloride anions, one of which displays a μ2-bridging function between the CuII and CdII ions. The extended structures of both complexes are distinctly lamellar. In particular, due to hydrogen–bonding interactions with participation of carboxylic groups, chloride atoms and secondary amino groups of the macrocycle, the electro-neutral molecules in crystal of I are arranged in chains running in the [101] direction; hydrogen bonding between the chains leads to layers parallel to the (101) plane. In crystal of II, polymeric chains running along the [101] direction are joined into layers parallel to the (101) plane via formation of Cu—Cl bonds and interchain hydrogen bonds. There are no hydrogen-bonding interactions between the layers and the three-dimensional structure of II is based on the weak C—H⋯O and C—H⋯Cl contacts.
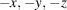
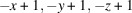
碳氧功能化Cu(双氮杂环)2+阳离子及其与CdCl2的异金属配位聚合物的合成与结构表征
配合物[3,10-二-(3-碳氧基-丙基)-1,3,5,8,10,12-六氮杂环-四癸烷-κ N 1,N 5,N 8,N 12]二氯铜(II), [CuCl2(C16H34N6O4)]或[Cu(H2 L)Cl2] (I)的不对称单元由一个中心对称大环CuII和一个氯阴离子组成。heterometallic复合聚[[aqua的组件——(μ3 - 3,10-bis - (3-carb-oxy-prop-yl) 1, 3, 5, 8, 10, 12-hexa-aza-cyclo-tetra-dec-ane -κ4 N 1, N 5 8 N, N 12:κ2 O, O ':κ2 O, O”)-μ-chlorido——铜(II)镉(二)1.25水合物],{[CuCd Cl2 (C16H32N6O4) (H2O)]·H2O} 1.25 N或{[CuCd (L) (H2O)氯]·H2O} 1.25 N(2)是(铜(L) (H2O))协调半个CdCl2单位通过deprotonated carb-oxy-lic macrocycle组,和四个水结晶mol-ecules部分入住率。在每个化合物中,CuII离子在赤道平面上由四个大环配体的二级N原子配位,其中大环配体采用能量最稳定的反式iii构象,两个相互反轴的配体分别在I和II中呈四边形细长的反式cun4cl2和反式cun4 (H2O)Cl八面体几何形状。CdII离子在II中的配位环境是由两个不同大环的去质子化碳氧基和两个氯阴离子形成的CdO4Cl2畸变八面体,其中一个在CuII和CdII离子之间表现出μ2桥接功能。这两种配合物的延伸结构都是明显的层状结构。特别是,由于大环的碳氧基、氯原子和仲氨基参与的氢键相互作用,I晶体中的电中性分子呈沿[101]方向排列的链;链之间的氢键形成平行于(101)平面的层。在II晶体中,沿[101]方向的聚合链通过Cu-Cl键和链间氢键的形成,连接成平行于(101)平面的层。层之间没有氢键相互作用,II的三维结构是基于弱的C-H⋯O和C-H⋯Cl接触。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Acta Crystallographica Section E: Crystallographic Communications
Chemistry-Chemistry (all)
CiteScore
1.90
自引率
0.00%
发文量
351
审稿时长
3 weeks
期刊介绍:
Acta Crystallographica Section E: Crystallographic Communications is the IUCr''s open-access structural communications journal. It provides a fast, simple and easily accessible publication mechanism for crystal structure determinations of inorganic, metal-organic and organic compounds. The electronic submission, validation, refereeing and publication facilities of the journal ensure rapid and high-quality publication of fully validated structures. The primary article category is Research Communications; these are peer-reviewed articles describing one or more structure determinations with appropriate discussion of the science.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


