Phenyl palladium(II) iodide complexes isolated after Sonogashira coupling of iodobenzenes with terminal alkynes
IF 0.6
Q4 CRYSTALLOGRAPHY
Acta Crystallographica Section E: Crystallographic Communications
Pub Date : 2025-06-01
DOI:10.1107/S2056989025004025
引用次数: 0
Abstract
The structure of four bis(triphenylphosphine)phenylpalladium(II) iodides isolated after completion of Sonogashira coupling reactions, in which the aryl iodide was used in slight excess, are reported.
The structures of four palladium complexes serendipitously isolated after palladium-catalyzed Sonogashira coupling of aryl iodides with terminal alkynes are reported, namely [3,5-bis(trifluoromethyl)phenyl]iodidobis(triphenylphosphane)palladium(II), [Pd(C8H3F6)I(C18H15P)2], 1, (3,5-dinitrophenyl)iodidobis(triphenylphosphane)palladium(II) ethyl acetate monosolvate, [Pd(C6H3N2O4)I(C18H15P)2]·C4H8O2, 2, (2,3,4-difluorophenyl)iodidobis(triphenylphosphane)palladium(II), [Pd(C6H2F3)I(C18H15P)2], 3, and (2,4,6-difluorophenyl)iodidobis(triphenylphosphane)palladium(II) dichloromethane disolvate, [Pd(C6H2F3)I(C18H15P)2]·2CH2Cl2, 4. These complexes were isolated as red/orange crystals that co-eluted with the organic products of each reaction during flash chromatography. These complexes are oxidative addition products ArPdI(PPh3)2 where Ar = 3,5-bis(trifluoromethyl)phenyl, 3,5-dinitrophenyl, 2,3,4-trifluorophenyl and 2,4,6-trifluoro-3,5-diiodophenyl. The isolation of these complexes provides some insight into the possible fate of the palladium catalyst.
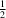

碘苯与末端炔Sonogashira偶联后分离的苯基碘化钯配合物。
报道了钯催化芳基碘化物与末端炔的Sonogashira偶联后偶然分离的四个钯配合物的结构,即[3,5-二-(三氟甲基)苯基]碘-二-(三苯基-磷)钯(II), [Pd(C8H3F6)I(C18H15P)2], 1,(3,5-二硝基-苯基)碘-二-(三苯基-磷)钯(II)乙酸乙酯单溶剂,[Pd(C6H3N2O4)I(C18H15P)2]·C4H8O2, 2,(2,3,4-二氟苯基)碘-二-(三苯基-磷)钯(II),[Pd(C6H2F3)I(C18H15P)2], 3,和(2,4,6-二氟苯基)碘-二-(三苯基膦)钯(II)二氯甲烷溶剂,[Pd(C6H2F3)I(C18H15P)2]·2ch2cl2,4。这些配合物被分离为红色/橙色晶体,在闪蒸色谱中与每个反应的有机产物共洗脱。这些配合物是氧化加成产物ArPdI(PPh3)2,其中Ar = 3,5-二(三氟甲基)苯基、3,5-二硝基苯基、2,3,4-三氟苯基和2,4,6-三氟-3,5-二碘苯基。这些络合物的分离为钯催化剂的可能命运提供了一些见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Acta Crystallographica Section E: Crystallographic Communications
Chemistry-Chemistry (all)
CiteScore
1.90
自引率
0.00%
发文量
351
审稿时长
3 weeks
期刊介绍:
Acta Crystallographica Section E: Crystallographic Communications is the IUCr''s open-access structural communications journal. It provides a fast, simple and easily accessible publication mechanism for crystal structure determinations of inorganic, metal-organic and organic compounds. The electronic submission, validation, refereeing and publication facilities of the journal ensure rapid and high-quality publication of fully validated structures. The primary article category is Research Communications; these are peer-reviewed articles describing one or more structure determinations with appropriate discussion of the science.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


