Crystal structure and Hirshfeld-surface analysis of 1-(4-fluorophenyl)-3,3-bis(methylsulfanyl)prop-2-en-1-one
IF 0.6
Q4 CRYSTALLOGRAPHY
Acta Crystallographica Section E: Crystallographic Communications
Pub Date : 2025-06-01
DOI:10.1107/S2056989025004189
引用次数: 0
Abstract
The crystal structure and a Hirshfeld-surface analysis of the chalcone derivative 1-(4-fluorophenyl)-3,3-bis(methylsulfanyl)prop-2-en-1-one are presented.
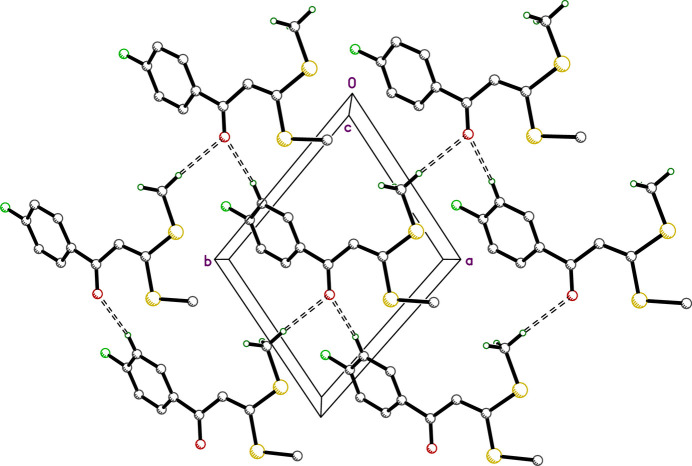
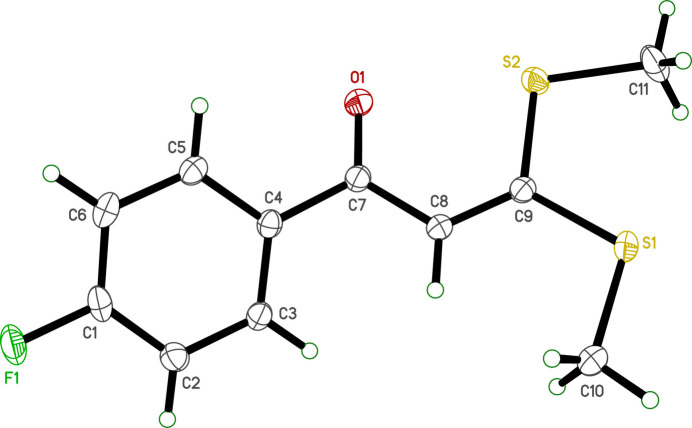
The title compound, C11H11FOS2, is a fluorinated chalcone derivative with potential applications in medicinal chemistry and functional materials. The molecular structure includes a planar 4-fluorophenyl ring linked by a carbonyl group and an ethenyl spacer to an approximately planar bis(methylsulfanyl) moiety (r.m.s. deviations from planarity are 0.0106 and 0.0315 Å, respectively). These planar groups are twisted relative to each other, subtending a dihedral angle of 32.23 (4)°. The crystal packing lacks classical hydrogen bonds or aromatic π-stacking, but molecules are connected through weaker C—H⋯O and C—H⋯S contacts into layers parallel to the ab plane and tapes extending along the b-axis direction. The 4-fluorophenyl groups on adjacent tapes interdigitate. Hirshfeld surface analysis shows that the majority (>90%) of intermolecular contacts involve hydrogen atoms.
1-(4-氟苯基)-3,3-二-(甲基磺基)- 2-烯-1- 1的晶体结构和赫希菲尔德表面分析。
标题化合物C11H11FOS2是一种氟化查尔酮衍生物,在药物化学和功能材料方面具有潜在的应用前景。分子结构包括一个平面的4-氟苯基环,由一个羰基和一个乙烯基间隔连接到一个近似平面的双(甲基-巯基)部分(相对于平面度的均方根偏差分别为0.0106和0.0315 Å)。这些平面群相互扭曲,呈32.23(4)°的二面角。晶体填料缺乏经典的氢键或芳香π堆积,但分子通过较弱的C-H⋯O和C-H⋯S接触连接成平行于ab平面的层和沿b轴方向延伸的带。相邻带上的4-氟苯基互指。Hirshfeld表面分析表明,分子间接触的大部分(约90%)涉及氢原子。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Acta Crystallographica Section E: Crystallographic Communications
Chemistry-Chemistry (all)
CiteScore
1.90
自引率
0.00%
发文量
351
审稿时长
3 weeks
期刊介绍:
Acta Crystallographica Section E: Crystallographic Communications is the IUCr''s open-access structural communications journal. It provides a fast, simple and easily accessible publication mechanism for crystal structure determinations of inorganic, metal-organic and organic compounds. The electronic submission, validation, refereeing and publication facilities of the journal ensure rapid and high-quality publication of fully validated structures. The primary article category is Research Communications; these are peer-reviewed articles describing one or more structure determinations with appropriate discussion of the science.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


