Targeting Ca2+-activated K+ channels in glioma
IF 4.2
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
Biochimica et biophysica acta. Molecular basis of disease
Pub Date : 2025-06-06
DOI:10.1016/j.bbadis.2025.167950
引用次数: 0
Abstract
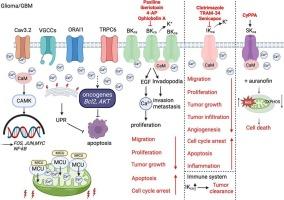
Glioma affects millions of people worldwide and there is a lack of effective therapies. Glioblastoma multiforme (GBM) is the most common and deadliest form of primary brain tumor in adults. Emerging evidence indicated that targeting ion channels may be a promising therapeutic approach for GBM. Altered expression and activity of the Ca2+- activated K+ (KCa) channels have been reported in GBM patients. Generally, large-conductance KCa (BKCa) channels and intermediate-conductance KCa (IKCa or SK4) channels are highly expressed in glioma samples compared to healthy control brain tissue. Analyzing TCGA database, the expression of KCNMB1 (encoding the BKCa channel protein) and KCNN4 (encoding the KCa3.1/IKCa protein) genes was upregulated, while KCNN1 (encoding the KCa2.1 protein) gene expression was downregulated in GBM patients grade IV compared to GBM patients grade I or II. The gene expression and activity of KCa channels may contribute to survival outcomes, by regulating cellular processes like cell proliferation and migration. Importantly, modulation of the activity of KCa channels reduced the proliferation and migration of GBM cells and suppressed glioma progression both in vivo and in vitro cell models for GBM. Herein, we aim to review how modulation of the activity of KCa channels impacts tumor development in terms of proliferation, cell death, invasion, metabolism and immune system in GBM.
在胶质瘤中靶向Ca2+激活的K+通道
神经胶质瘤影响着全世界数百万人,目前缺乏有效的治疗方法。多形性胶质母细胞瘤(GBM)是成人中最常见和最致命的原发性脑肿瘤。新出现的证据表明,靶向离子通道可能是一种很有前途的治疗GBM的方法。Ca2+激活的K+ (KCa)通道的表达和活性改变在GBM患者中有报道。一般来说,与健康对照脑组织相比,大电导KCa (BKCa)通道和中电导KCa (IKCa或SK4)通道在胶质瘤样本中高度表达。分析TCGA数据库发现,与ⅰ级或ⅱ级GBM患者相比,ⅳ级GBM患者中编码BKCa通道蛋白的KCNMB1和编码KCa3.1/IKCa蛋白的KCNN4基因表达上调,而编码KCa2.1蛋白的KCNN1基因表达下调。KCa通道的基因表达和活性可能通过调节细胞增殖和迁移等细胞过程而影响存活结果。重要的是,在体内和体外GBM细胞模型中,KCa通道活性的调节减少了GBM细胞的增殖和迁移,抑制了胶质瘤的进展。在此,我们旨在回顾KCa通道活性的调节如何在GBM中影响肿瘤的增殖、细胞死亡、侵袭、代谢和免疫系统。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
12.30
自引率
0.00%
发文量
218
审稿时长
32 days
期刊介绍:
BBA Molecular Basis of Disease addresses the biochemistry and molecular genetics of disease processes and models of human disease. This journal covers aspects of aging, cancer, metabolic-, neurological-, and immunological-based disease. Manuscripts focused on using animal models to elucidate biochemical and mechanistic insight in each of these conditions, are particularly encouraged. Manuscripts should emphasize the underlying mechanisms of disease pathways and provide novel contributions to the understanding and/or treatment of these disorders. Highly descriptive and method development submissions may be declined without full review. The submission of uninvited reviews to BBA - Molecular Basis of Disease is strongly discouraged, and any such uninvited review should be accompanied by a coverletter outlining the compelling reasons why the review should be considered.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


